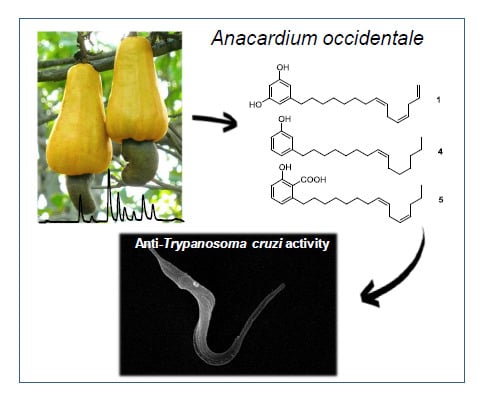
Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins
Abstract
:1. Introduction
2. Results
2.1. The Cashew Nut Isolated Compounds
2.2. Trypanosoma cruzi Recombinant Sirtuins In Vitro Deacetylase Activity
2.3. Anti-Trypanosoma cruzi In Vitro Evaluations
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction
4.4. Purification of the Dichloromethane Extract of Cashew Using MPLC and Semi-Preparative HPLC
4.5. Recombinant TcSir2rp1 and TcSir2rp3
4.6. Deacetylase Activity Assay
4.7. Host Cell Toxicity
4.8. Parasites
4.9. Activity Against Trypomastigotes
4.10. In Vitro T. cruzi Infection Assay
4.11. Flow Cytometry Analysis
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanaway, J.D.; Roth, G. The Burden of Chagas Disease. Global Heart 2015, 10, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Soriano-Arandes, A.; Angheben, A.; Serre-Delcor, N.; Treviño-Maruri, B.; Gómezi Prat, J.; Jackson, Y. Control and management of congenital Chagas disease in Europe and other non-endemic countries: Current policies and practices. Trop. Med. Int. Health 2016, 21, 590–596. [Google Scholar] [CrossRef]
- Coura, J.R.; De Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. I. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Morilla, M.J.; Romero, E.L. Nanomedicines against Chagas disease: An update on therapeutics, prophylaxis and diagnosis. Nanomedicine 2015, 10, 465–481. [Google Scholar] [CrossRef]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.J.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef]
- Brachmann, C.B.; Sherman, J.M.; Devine, S.E.; Cameron, E.E.; Pillus, L.; Boeke, J.D. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Gene. Dev. 1995, 9, 2888–2902. [Google Scholar] [CrossRef] [PubMed]
- Religa, A.A.; Waters, A.P. Sirtuins of parasitic protozoa: In search of function(s). Mol. Biochem. Parasit. 2012, 185, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Moretti, N.S.; da Silva Augusto, L.; Clemente, T.M.; Antunes, R.P.P.; Yoshida, N.; Schenkman, S.; Torrecilhas, A.C.; Cano, M.I.N. Characterization of Trypanosoma cruzi Sirtuins as Possible Drug Targets for Chagas Disease. Antimicrob. Agents Chemother. 2015, 59, 4669–4679. [Google Scholar] [CrossRef]
- Ritagliati, C.; Alonso, V.L.; Manarin, R.; Cribb, P.; Serra, E.C. Overexpression of Cytoplasmic TcSIR2RP1 and Mitochondrial TcSIR2RP3 Impacts on Trypanosoma cruzi Growth and Cell Invasion. PLoS Neglect. Trop. D. 2015, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Alsford, S.; Kawahara, T.; Isamah, C.; Horn, D. A sirtuin in the African trypanosome is involved in both DNA repair and telomeric gene silencing but is not required for antigenic variation. Mol. Microbiol. 2007, 63, 724–736. [Google Scholar] [CrossRef]
- García-Salcedo, J.A.; Gijón, P.; Nolan, D.P.; Tebabi, P.; Pays, E. A chromosomal SIR2 homologue with both histone NAD-dependent ADP-ribosyltransferase and deacetylase activities is involved in DNA repair in Trypanosoma brucei. EMBO J. 2003, 22, 5851–5862. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.P.; Silva, C.V.; Bastos, T.M.; Guimarães, E.T.; Figueira, C.P.; Smirlis, D.; Azevedo, W.F. Anti-Trypanosoma cruzi activity of nicotinamide. Acta Trop. 2012, 122, 224–229. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Reignault, L.C.; Huber, K.; Bracher, F.; de Souza, W.; de Carvalho, T.M.U. Inhibition of NAD+-dependent histone deacetylases (sirtuins) causes growth arrest and activates both apoptosis and autophagy in the pathogenic protozoan Trypanosoma cruzi. Parasitology 2014, 141, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, B.; Gazanion, E.; Grentzinger, T. Functional divergence of SIR2 orthologs between trypanosomatid parasites. Mol. Biochem. Parasit. 2016, 27, 96–101. [Google Scholar] [CrossRef]
- Zheng, W. Sirtuins as emerging anti-parasitic targets. Eur. J. Med. Chem. 2013, 59, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.; Coron, R.P.; KongThoo Lin, P.; Costa, D.M.; Perez-Cabezas, B.; Tavares, J.; Roura-Ferrer, M.; Ramos, I.; Ronin, C.; Major, L.L.; et al. Inhibitors of Trypanosoma cruzi Sir2 related protein 1 as potential drugs against Chagas disease. PLoS Neglect. Trop. D. 2018, 12, e0006180. [Google Scholar] [CrossRef]
- Sacconnay, L.; Angleviel, M.; Randazzo, G.M.; Marçal Ferreira Queiroz, M.; Ferreira Queiroz, E.; Wolfender, J.L.; Carrupt, P.A.; Nurisso, A. Computational Studies on Sirtuins from Trypanosoma cruzi: Structures, Conformations and Interactions with Phytochemicals. PLoS Neglect. Trop. D. 2014, 8. [Google Scholar] [CrossRef]
- Mazzetto, S.E.; Lomonaco, D.; Mele, G. Oleo da castanha de caju: Oportunidades e desafios no contexto do desenvolvimento e sustentabilidade industrial. Quimica Nova 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Swaminathan, V.; Ranganathan, A.; Kundu, T.K. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 2003, 278, 19134–19140. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M.; Yamagiwa, Y.; Mera, H.; Tokushima, K.; Ohta, S.; Kamikawa, T. Structure-antibacterial activity relationships of anacardic acids. J. Agric. Food. Chem. 1993, 41, 1016–1019. [Google Scholar] [CrossRef]
- Mai, A.; Rotili, D.; Tarantino, D.; Ornaghi, P.; Tosi, F.; Vicidomini, C.; Sbardella, G.; Nebbioso, A.; Miceli, M.; Altucci, L.; et al. Small-molecule inhibitors of histone acetyltransferase activity: Identification and biological properties. J. Med. Chem. 2006, 49, 6897–6907. [Google Scholar] [CrossRef]
- Oliveira, M.S.C.; de Morais, S.M.; Magalhães, D.V.; Batista, W.P.; Vieira, Í.G.P.; Craveiro, A.A.; de Manezes, J.E.S.A.; Carvalho, A.F.U.; de Lima, G.P.G. Antioxidant, larvicidal and antiacetylcholinesterase activities of cashew nut shell liquid constituents. Acta Trop. 2011, 117, 165–170. [Google Scholar] [CrossRef]
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M. Anacardic Acid (6-Pentadecylsalicylic Acid) Inhibits Tumor Angiogenesis by Targeting Src/FAK/Rho GTPases Signaling Pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Kubo, I.; Kinst-Hori, I.; Yokokawa, Y. Tyrosinase inhibitors from Anacardium occidentale fruits. J. Nat. Prod. 1994, 57, 545–551. [Google Scholar]
- Kubo, I.; Komatsu, S.; Ochi, M. Molluscicides from the cashew Anacardium occidentale and their large-scale isolation. J. Agric. Food. Chem. 1986, 34, 970–973. [Google Scholar] [CrossRef]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel Method for Isolation of Major Phenolic Constituents from Cashew (Anacardium occidentale L.) Nut Shell Liquid. J. Agric. Food. Chem. 2001, 49, 2548–2551. [Google Scholar] [PubMed]
- Challal, S.; Queiroz, E.; Debrus, B.; Kloeti, W.; Guillarme, D.; Gupta, M.; Wolfender, J.-L. Rational and Efficient Preparative Isolation of Natural Products by MPLC-UV-ELSD based on HPLC to MPLC Gradient Transfer. Planta Med. 2015, 81, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Silva, K.; Araujo, H.; Vieira, I.; Alves, D.; Fontenelle, R.; Silva, A. Anacardic Acid Constituents from Cashew Nut Shell Liquid: NMR Characterization and the Effect of Unsaturation on Its Biological Activities. Pharmaceuticals 2017, 10, 31. [Google Scholar] [CrossRef]
- Alvarenga, T.A.; de Oliveira, P.F.; de Souza, J.M.; Tavares, D.C.; Andrade e Silva, M.L.; Cunha, W.R.; Groppo, M.; Januário, A.H.; Magalhães, L.G.; Pauletti, P.M. Schistosomicidal Activity of Alkyl-phenols from the Cashew Anacardium occidentale against Schistosoma mansoni Adult Worms. J. Agric. Food. Chem. 2016, 64, 8821–8827. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-X.; Hu, Y.-H.; Yang, M.-H.; Liu, F.-J.; Qiu, L.; Zhou, X.-W.; Chen, Q.-X. Irreversible Competitive Inhibitory Kinetics of Cardol Triene on Mushroom Tyrosinase. J. Agric. Food. Chem. 2010, 58, 12993–12998. [Google Scholar] [CrossRef] [PubMed]
- Attanasi, O.A.; Berretta, S.; Fiani, C.; Filippone, P.; Mele, G.; Saladino, R. Synthesis and reactions of nitro derivatives of hydrogenated cardanol. Tetrahedron 2006, 62, 6113–6120. [Google Scholar] [CrossRef]
- Pereira, J.M.; Severino, R.P.; Vieira, P.C.; Fernandes, J.B.; da Silva, M.F.G.F.; Zottis, A.; Andricopulo, A.D.; Oliva, G.; Corrêa, A.G. Anacardic acid derivatives as inhibitors of glyceraldehyde-3-phosphate dehydrogenase from Trypanosoma cruzi. Bioorgan. Med. Chem. 2008, 16, 8889–8895. [Google Scholar] [CrossRef] [PubMed]
- Maya, J.D.; Cassels, B.K.; Iturriaga-Vásquez, P.; Ferreira, J.; Faúndez, M.; Galanti, N.; Ferreira, A.; Morello, A. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Phys. 2007, 146, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Compounds | TcSir2rp1 | TcSir2rp3 | Trypomastigote | Amastigote | hFIB | SI e |
|---|---|---|---|---|---|---|
| EC50 ± SEM (µM) a | EC50 ± SEM (µM) a | EC50 ± SEM (µM) b | EC50 ± SEM (µM) c | CC50 ± SEM (µM) d | ||
| 1 | NA | NA | 23.36 ± 0.12 | 11.75 ± 0.40 | 47.17 ± 0.10 | 4 |
| 2 | 31.40 ± 2.33 | NA | 12.25 ± 0.25 | 14.70 ± 3.27 | 53.70 ± 7.08 | 4 |
| 3 | NA | NA | NA | 46.98 ± 1.97 | >100 | >2 |
| 4 | NA | NA | 43.52 ± 2.32 | 15.28 ± 2.00 | 45.47 ± 0.27 | 3 |
| 5 | 23.35 ± 2.51 | 6.08 ± 1.16 | NA | 41.67 ± 5.53 | >100 | >2 |
| 6 | 16.16 ± 4.88 | 7.41 ± 0.65 | NA | 42.39 ± 5.11 | >100 | >2 |
| Benznidazole | 11.40 ± 1.09 | 3.13 ± 0.87 | >100 | >32 |
| EC50 ± SD (µM) | |||
|---|---|---|---|
| Compounds | Alone | Combination | CI ± SD (µM) |
| 2 | 12.15 ± 6.46 | 2.69 ± 1.13 | 1.35 ± 0.04 |
| Benznidazole | 2.17 ± 0.09 | 2.45 ± 0.34 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matutino Bastos, T.; Mannochio Russo, H.; Silvio Moretti, N.; Schenkman, S.; Marcourt, L.; Gupta, M.P.; Wolfender, J.-L.; Ferreira Queiroz, E.; Botelho Pereira Soares, M. Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins. Molecules 2019, 24, 1299. https://doi.org/10.3390/molecules24071299
Matutino Bastos T, Mannochio Russo H, Silvio Moretti N, Schenkman S, Marcourt L, Gupta MP, Wolfender J-L, Ferreira Queiroz E, Botelho Pereira Soares M. Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins. Molecules. 2019; 24(7):1299. https://doi.org/10.3390/molecules24071299
Chicago/Turabian StyleMatutino Bastos, Tanira, Helena Mannochio Russo, Nilmar Silvio Moretti, Sergio Schenkman, Laurence Marcourt, Mahabir Prashad Gupta, Jean-Luc Wolfender, Emerson Ferreira Queiroz, and Milena Botelho Pereira Soares. 2019. "Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins" Molecules 24, no. 7: 1299. https://doi.org/10.3390/molecules24071299
APA StyleMatutino Bastos, T., Mannochio Russo, H., Silvio Moretti, N., Schenkman, S., Marcourt, L., Gupta, M. P., Wolfender, J. -L., Ferreira Queiroz, E., & Botelho Pereira Soares, M. (2019). Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins. Molecules, 24(7), 1299. https://doi.org/10.3390/molecules24071299