Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species
Abstract
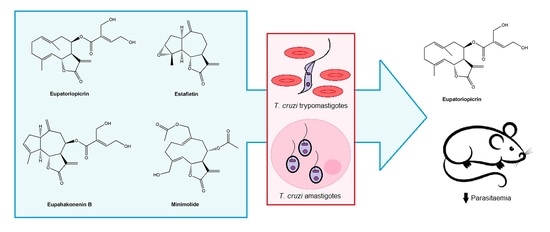
:1. Introduction
2. Results
2.1. Isolation and Identification of the Sesquiterpene Lactones from Asteraceae Species
2.2. In Vitro Anti-T. cruzi Activity
2.3. Cytotoxicity
2.4. In Vivo Assay
2.5. Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sesquiterpene Lactones Isolation
4.3. Parasites
4.4. Activity Assay on Bloodstream T. Cruzi Trypomastigotes
4.5. Activity Assay on Intracellular T. Cruzi Amastigotes
4.6. Cytotoxicity
4.7. In Vivo Trypanocidal Activity
4.8. Histopathological Analysis
4.9. Ethic Statement
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan American Health Organization (PAHO). Chagas Disease, 2019. Available online: https://www.paho.org/hq/index.php?option=com_topics&view=article&id=10&Itemid=40743&lang=en (accessed on 20 February 2020).
- World Health Organization (WHO). Chagas Disease (American Trypanosomiasis). 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 1 March 2020).
- Andrade, D.V.; Gollob, K.J.; Dutra, W.O. Acute Chagas disease: New global challenges for an old neglected disease. PLoS Negl. Trop. Dis. 2014, 8, e3010. [Google Scholar] [CrossRef]
- Espuelas, S.; Plano, D.; Nguewa, P.; Font, M.; Palop, J.A.; Irache, J.M.; Sanmartin, C. Innovative lead compounds and formulation strategies as newer kinetoplastid therapies. Curr. Med. Chem. 2012, 19, 4259–4288. [Google Scholar] [CrossRef]
- Schmidt, T.; Khalid, S.; Romanha, A.; Alves, T.; Biavatti, M.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef]
- Sülsen, V.; Martino, V. Overview. In Sesquiterpene Lactones. Advances in their Chemistry and Biological Aspects; Sülsen, V., Martino, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar]
- Jerapan, K.; Sudaratana, R. Antimalarial qinghaosu/artemisinin: The therapy worthy of a nobel prize. Asian Pac. J. Trop. Biomed. 2016, 6, 371–375. [Google Scholar]
- Soejarto, D.D. Ethnobotany of Stevia and Stevia rebaudiana. In Stevia. The genus Stevia; Kinghorn, D.A., Ed.; Taylor and Francis: London, UK; New York, NY, USA, 2002; pp. 40–67. [Google Scholar]
- Hernandez, L.R.; De Riscala, E.C.; Catalán, C.A.N.; Diaz, J.G.; Herz, W. Sesquiterpene lactones and others constituents of Stevia maimarensis and Synedrellopsis grisebachii. Phytochemistry 1996, 42, 681–684. [Google Scholar] [CrossRef]
- De Heluani, C.; Lampasona, M.; Catalan, C.; Goedken, V.; Gutierrez, A.; Gutierrez, A.; Herz, W. Guaianolides, heliangolides and other constituents from Stevia alpina. Phytochemistry 1989, 28, 1931–1935. [Google Scholar] [CrossRef]
- Hernández, L.R.; Catalán, C.A.N.; Cerda García Rojas, C.M.; Josdeph-Nathan, J. Guaianolides from Stevia gilliesi. Nat. Prod. Res. 1995, 6, 215–221. [Google Scholar]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Zapata-Martínez, J.; Sánchez-Toranzo, G.; Chaín, F.; Catalán, C.A.; Bühler, M.I. Effect of guaianolides in the meiosis reinitiation of amphibian oocytes. Zygote 2017, 25, 10–16. [Google Scholar] [CrossRef]
- Michalak, B.; Piwowarski, J.P.; Granica, S.; Waltenberger, B.; Atanasov, A.G.; Khan, S.Y.; Breuss, J.M.; Uhrin, P.; Żyżyńska-Granica, B.; Stojakowska, A.; et al. Eupatoriopicrin inhibits pro-inflammatory functions of neutrophils via suppression of IL-8 and TNF-alpha production and p38 and ERK 1/2 MAP kinases. J. Nat. Prod. 2019, 82, 375–385. [Google Scholar] [CrossRef]
- Julianti, T.; Hata, Y.; Zimmermann, S.; Kaiser, M.; Hamburger, M.; Adams, M. Antitrypanosomal sesquiterpene lactones from Saussurea costus. Fitoterapia 2011, 82, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Fabian, L.; Sülsen, V.; Frank, F.; Cazorla, S.; Malchiodi, E.; Martino, V. In silico study of structural and geometrical requirements of natural sesquiterpene lactones with trypanocidal activity. Mini Rev. Med. Chem. 2013, 13, 407–1414. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.B.; Owiti, A.; Barbosa, W. Pharmacology of Mikania genus: A systematic review. Pharm. Rev. 2018, 12, 230–237. [Google Scholar]
- Cuenca, M.R.; Borkosky, S.; Catalán, C.A.N.; Goedken, V.L.; Díaz, J.G.; Herz, W. Sesquiterpene lactones of Mikania minima. Phytochemistry 1993, 32, 1509–1513. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Lizarraga, E.F.; Elso, O.G.; Cerny, N.; Sanchez Alberti, A.; Bivona, A.E.; Malchiodi, E.L.; Cazorla, S.I.; Catalán, C.A.N. Activity of estafietin and analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 2019, 24, 1209. [Google Scholar] [CrossRef] [Green Version]
- Risso, M.; Garbarino, G.; Mocetti, E.; Campetella, O.; Gonzalez Cappa, S.; Buscaglia, C.; Leguizamon, M. Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J. Infect. Dis. 2004, 189, 2250–2259. [Google Scholar] [CrossRef] [Green Version]
- Burgos, J.; Risso, M.; Brenière, S.; Barnabé, C.; Campetella, O.; Leguizamón, M. Differential distribution of genes encoding the virulence factor trans-sialidase along Trypanosoma cruzi Discrete typing units. PLoS ONE 2013, 8, e58967. [Google Scholar] [CrossRef]
- Alba Soto, C.; Mirkin, G.; Solana, M.; González Cappa, S. Trypanosoma cruzi infection modulates in vivo expression of major histocompatibility complex class II molecules on antigen-presenting cells and T-cell stimulatory activity of dendritic cells in a strain-dependent manner. Infect. Immun. 2003, 71, 1194–1199. [Google Scholar] [CrossRef] [Green Version]
- Segura, E.L.; Cardoni, R.L.; Búa, J.; Rottenberg, M.E.; Bontempi, E.J.; Esteva, M.I.; Carlomagno, M.A.; De Titto, E.H.; Ruiz, A.M. Molecular and immunologic bases for the development of a vaccine against Chagas disease. Med. B Aires 1989, 49, 203–209. [Google Scholar]
- Vieira, J.; Távora, F.; Sobral, M.; Vasconcelos, G.; Almeida, G.; Fernandes, J.; da Escóssia Marinho, L.; de Mendonça Trompieri, D.; De Souza Neto, J.; Mejia, J. Chagas cardiomyopathy in Latin America review. Curr. Cardiol. Rep. 2019, 21, 8. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Frank, M.; Cazorla, S.; Anesini, C.; Malchiodi, E.; Roser Vila, B.; Muschietti, L.; Martino, V. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia sprengel (Asteraceae). Antimicrob. Agents Chemother. 2008, 52, 2415–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sülsen, V.P.; Cazorla, S.I.; Frank, F.M.; Laurella, L.C.; Muschietti, L.V.; Catalán, C.A.; Martino, V.; Malchiodi, E. Natural terpenoids from Ambrosia species are active in vitro and in vivo against human pathogenic trypanosomatids. PLoS Negl. Trop. Dis. 2013, 7, e2494. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.F.; Batista, D.G.; De Araújo, J.S.; Batista, M.M.; Lionel, J.; de Souza, E.M.; Hammer., E.R.; da Silva, P.B.; De Mieri, M.; Adams, M.; et al. Activities of psilostachyin A and cynaropicrin against Trypanosoma cruzi in vitro and in vivo. Antimicrob. Agents Chemother. 2013, 57, 5307–5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milagre, M.; Branquinho, R.; Gonçalves, M.; De Assis, G.; De Oliveira, M.; Reis, L.; Saúde-Guimarães, D.A.; De Lana, M. Activity of the sesquiterpene lactone goyazensolide against Trypanosoma cruzi in vitro and in vivo. Parasitology 2020, 147, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Postan, M.; Lucas, P.; Gress, R.; Tarleton, R. TGF-beta regulates pathology but not tissue CD8 + T cell dysfunction during experimental Trypanosoma cruzi infection. Eur. J. Immunol. 2007, 37, 2764–2771. [Google Scholar] [CrossRef]
Sample Availability: Samples of selected compounds are available from the authors. |






| Sesquiterpene Lactones | Selectivity Index (SI) | |
|---|---|---|
| Trypomastigotes | Amastigotes | |
| Eupatoriopicrin | 12.9 | 40.6 |
| Estafietin | 6.8 | 7.3 |
| Eupahakonenin B | 10.4 | 3.8 |
| Minimolide | 12.8 | 10.7 |
| Benznidazole | 18.6 | 87.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elso, O.G.; Bivona, A.E.; Sanchez Alberti, A.; Cerny, N.; Fabian, L.; Morales, C.; Catalán, C.A.N.; Malchiodi, E.L.; Cazorla, S.I.; Sülsen, V.P. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules 2020, 25, 2014. https://doi.org/10.3390/molecules25092014
Elso OG, Bivona AE, Sanchez Alberti A, Cerny N, Fabian L, Morales C, Catalán CAN, Malchiodi EL, Cazorla SI, Sülsen VP. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules. 2020; 25(9):2014. https://doi.org/10.3390/molecules25092014
Chicago/Turabian StyleElso, Orlando G., Augusto E. Bivona, Andrés Sanchez Alberti, Natacha Cerny, Lucas Fabian, Celina Morales, César A. N. Catalán, Emilio L. Malchiodi, Silvia I. Cazorla, and Valeria P. Sülsen. 2020. "Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species" Molecules 25, no. 9: 2014. https://doi.org/10.3390/molecules25092014
APA StyleElso, O. G., Bivona, A. E., Sanchez Alberti, A., Cerny, N., Fabian, L., Morales, C., Catalán, C. A. N., Malchiodi, E. L., Cazorla, S. I., & Sülsen, V. P. (2020). Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules, 25(9), 2014. https://doi.org/10.3390/molecules25092014