On the Enhanced Accuracy of Kinetic Curve Building in Supercritical Fluid Extraction from Aroma Plants Using a New 3D-Printed Extract Collection Device
Abstract
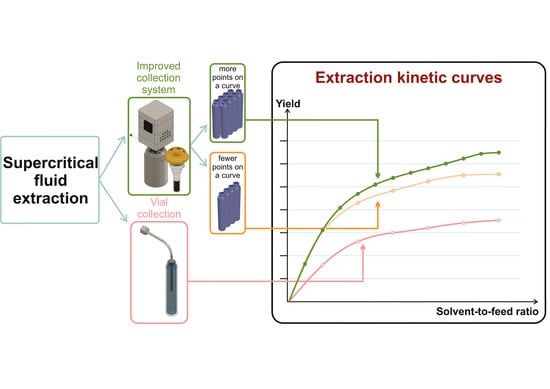
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Supercritical Fluid Extraction Procedure
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020. [Google Scholar] [CrossRef] [Green Version]
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agro-industrial waste: challenges, potentialities, and perspectives on emerging approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Payán, M. Liquid-Phase microextraction and electromembrane extraction in millifluidic devices: A tutorial. Anal. Chim. Acta 2019, 1080, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Alexovič, M.; Horstkotte, B.; Solich, P.; Sabo, J. Automation of static and dynamic non-dispersive liquid phase microextraction. Part 2: Approaches based on impregnated membranes and porous supports. Anal. Chim. Acta 2016, 907, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Alexovič, M.; Dotsikas, Y.; Bober, P.; Sabo, J. Achievements in robotic automation of solvent extraction and related approaches for bioanalysis of pharmaceuticals. J. Chromatogr. B 2018, 1092, 402–421. [Google Scholar] [CrossRef]
- Martínez, J.; Aguiar, A.C. de Analytical supercritical fluid extraction. In Analytical Separation Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1659–1680. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies - a practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef]
- Martinez, J.L. Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2007; 424p. [Google Scholar]
- del Valle, J.M. Extraction of natural compounds using supercritical CO2: Going from the laboratory to the industrial application. J. Supercrit. Fluids 2015, 96, 180–199. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K. Supercritical fluids and the food industry. Compr. Rev. Food Sci. Food Saf. 2002, 1, 33–44. [Google Scholar] [CrossRef]
- Matsuyama, K. Supercritical fluid processing for metal-organic frameworks, porous coordination polymers, and covalent organic frameworks. J. Supercrit. Fluids 2018, 134, 197–203. [Google Scholar] [CrossRef]
- McHardy, J.; Sawan, S.P. Supercritical fluid cleaning: fundamentals, technology and applications; Noyes: Westwood, NJ, USA, 1998. [Google Scholar]
- Smirnova, I.; Gurikov, P. Aerogel production: current status, research directions, and future opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Jinno, K. Hyphenated Techniques in Supercritical Fluid Chromatography and Extraction; School of materials science; Toyohashi university of technology: Toyohashi, Japan, 1992. [Google Scholar]
- Zoccali, M.; Donato, P.; Mondello, L. Recent advances in the coupling of carbon dioxide-based extraction and separation techniques. TrAC Trends Anal. Chem. 2019, 116, 158–165. [Google Scholar] [CrossRef]
- del Sánchez-Camargo, A.P.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. On-line coupling of supercritical fluid extraction and chromatographic techniques. J. Sep. Sci. 2017, 40, 213–227. [Google Scholar]
- del Sánchez-Camargo, A.P.; Parada-Alonso, F.; Ibáñez, E.; Cifuentes, A. Recent applications of on-line supercritical fluid extraction coupled to advanced analytical techniques for compounds extraction and identification. J. Sep. Sci. 2019, 42, 243–257. [Google Scholar]
- Sakai, M.; Hayakawa, Y.; Funada, Y.; Ando, T.; Fukusaki, E.; Bamba, T. Development of a practical online supercritical fluid extraction–supercritical fluid chromatography/mass spectrometry system with an integrated split-flow method. J. Chromatogr. A 2019, 1592, 161–172. [Google Scholar] [CrossRef]
- Lanças, F.M. Supercritical fluid techniques coupled with chromatographic techniques. In Multidimensional Chromatography; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 135–150. [Google Scholar]
- Abrahamsson, V.; Rodriguez-Meizoso, I.; Turner, C. Supercritical fluid extraction of lipids from linseed with on-line evaporative light scattering detection. Anal. Chim. Acta 2015, 853, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, V.; Jumaah, F.; Turner, C. Continuous multicomponent quantification during supercritical fluid extraction applied to microalgae using in-line UV/Vis absorption spectroscopy and on-line evaporative light scattering detection. J. Supercrit. Fluids 2018, 131, 157–165. [Google Scholar] [CrossRef]
- Abrahamsson, V.; Cunico, L.P.; Andersson, N.; Nilsson, B.; Turner, C. Multicomponent inverse modeling of supercritical fluid extraction of carotenoids, chlorophyll A, ergosterol and lipids from microalgae. J. Supercrit. Fluids 2018, 139, 53–61. [Google Scholar] [CrossRef]
- Thompson, P.G.; Taylor, L.T.; Richter, B.E.; Porter, N.L.; Ezzell, J.L. Trapping efficiencies of various collection solvents after supercritical fluid extraction. J. High Resolut. Chromatogr. 1993, 16, 713–716. [Google Scholar] [CrossRef]
- Thompson, P.G.; Taylor, L.T. Liquid trapping after supercritical fluid extraction with modified carbon dioxide. J. High Resolut. Chromatogr. 1994, 17, 759–764. [Google Scholar] [CrossRef]
- McDaniel, L.H.; Taylor, L.T. Modification of the collection solvent to enhance liquid trapping efficiencies after supercritical fluid extraction. J. Chromatogr. Sci. 1999, 37, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.M.; Ravey, R.M.; Houck, R.K.; Ashraf-Khorassani, M. Considerations in off-line collection after SFE. Fresenius, J. Anal. Chem. 1992, 344, 517–520. [Google Scholar] [CrossRef]
- Björklund, E.; Mathiasson, L.; Persson, P.; Järemo, M. Collection capacity of a solid phase trap in supercritical fluid extraction for the extraction of lipids from a model fat sample. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 2133–2143. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Valverde-García, A. Evaluation of different solid-phase traps for automated collection and clean-up in the analysis of multiple pesticides in fruits and vegetables after supercritical fluid extraction. J. Chromatogr. A 1997, 765, 69–84. [Google Scholar] [CrossRef]
- Moore, W.N.; Taylor, L.T. Solid phase trapping mechanisms involved in the supercritical fluid extraction of digitalis glycosides with modified carbon dioxide. Anal. Chem. 1995, 67, 2030–2036. [Google Scholar] [CrossRef]
- Han, Y.; Ren, L.; Xu, K.; Yang, F.; Li, Y.; Cheng, T.; Kang, X.; Xu, C.; Shi, Q. Supercritical fluid extraction with carbon nanotubes as a solid collection trap for the analysis of polycyclic aromatic hydrocarbons and their derivatives. J. Chromatogr. A 2015, 1395, 1–6. [Google Scholar] [CrossRef]
- Hartonen, K.; Bøwadt, S.; Hawthorne, S.B.; Riekkola, M.-L. Supercritical fluid extraction with solid-phase trapping of chlorinated and brominated pollutants from sediment samples. J. Chromatogr. A 1997, 774, 229–242. [Google Scholar] [CrossRef]
- Ashraf-Khorassani, M.; Houck, R.K.; Levy, J.M. Cryogenically Cooled Adsorbent Trap for Off-Line Supercritical Fluid Extraction. J. Chromatogr. Sci. 1992, 30, 361–366. [Google Scholar] [CrossRef]
- Lojková, L.; Sedláková, J.; Kubáň, V. A solvent recirculation trapping device for supercritical fluid extraction of polyaromatic hydrocarbons. J. Sep. Sci. 2005, 28, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.; Wyllie, S.G.; Cornwell, C.P.; Tronson, D. Supercritical Fluid Extraction (SFE) of Monoterpenes from the Leaves of Melaleuca alternifolia (Tea Tree). Molecules 2001, 6, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Grosso, C.; Coelho, J.P.; Pessoa, F.L.P.; Fareleira, J.M.N.A.; Barroso, J.G.; Urieta, J.S.; Palavra, A.F.; Sovová, H. Mathematical modelling of supercritical CO2 extraction of volatile oils from aromatic plants. Chem. Eng. Sci. 2010, 65, 3579–3590. [Google Scholar] [CrossRef]
- Sovová, H. Modeling the supercritical fluid extraction of essential oils from plant materials. J. Chromatogr. A 2012, 1250, 27–33. [Google Scholar] [CrossRef]
- Sovová, H. Steps of supercritical fluid extraction of natural products and their characteristic times. J. Supercrit. Fluids 2012, 66, 73–79. [Google Scholar] [CrossRef]
- Núñez, G.A.; Gelmi, C.A.; del Valle, J.M. Simulation of a supercritical carbon dioxide extraction plant with three extraction vessels. Comput. Chem. Eng. 2011, 35, 2687–2695. [Google Scholar] [CrossRef]
- Ibáñez, E.; Oca, A.; de Murga, G.; López-Sebastián, S.; Tabera, J.; Reglero, G. Supercritical Fluid Extraction and Fractionation of Different Preprocessed Rosemary Plants. J. Agric. Food Chem. 1999, 47, 1400–1404. [Google Scholar] [CrossRef]
- Ramsey, E.D. (Ed.) Analytical Supercritical Fluid Extraction Techniques; Springer: Dordrecht, The Netherlands, 1998; ISBN 978-94-010-6076-9. [Google Scholar]
- Ustinovich, K.B.; Prokopchuk, D.I.; Pokrovskiy, O.I.; Parenago, O.O.; Lunin, V.V. A Small Volume Cyclone Separator for Supercritical Fluid Extraction. Russ. J. Phys. Chem. B 2018, 12, 1306–1309. [Google Scholar] [CrossRef]
- García-Risco, M.R.; Hernández, E.J.; Vicente, G.; Fornari, T.; Señoráns, F.J.; Reglero, G. Kinetic study of pilot-scale supercritical CO2 extraction of rosemary (Rosmarinus officinalis) leaves. J. Supercrit. Fluids 2011, 55, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Pokrovskiy, O.I.; Prokopchuk, D.I.; Bagatelia, S.A.; Pokryshkin, S.A.; Kostenko, M.O.; Parenago, O.O.; Markolia, A.; Lunin, V.V. Comparison of Laurus nobilis extracts composition obtained by microwave extraction, supercritical fluid extraction and steam distillation. Chem. Plant Raw Mater. 2019, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Sovová, H.; Nobre, B.P.; Palavra, A. Modeling of the Kinetics of Supercritical Fluid Extraction of Lipids from Microalgae with Emphasis on Extract Desorption. Materials 2016, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlić, B.; Bera, O.; Vidović, S.; Ilić, L.; Zeković, Z. Extraction kinetics and ANN simulation of supercritical fluid extraction of sage herbal dust. J. Supercrit. Fluids 2017, 130, 327–336. [Google Scholar] [CrossRef]
- Denis, P.; Konstantin, U.; Oleg, P. A simple extract collection system for lab-scale supercritical fluid extraction with high trapping efficiency. Submitted to HardwareX. Available online: https://www.sciencedirect.com/journal/hardwarex (accessed on 1 January 2020).
Sample Availability: Samples of the compounds are not available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokopchuk, D.; Pokrovskiy, O. On the Enhanced Accuracy of Kinetic Curve Building in Supercritical Fluid Extraction from Aroma Plants Using a New 3D-Printed Extract Collection Device. Molecules 2020, 25, 2008. https://doi.org/10.3390/molecules25092008
Prokopchuk D, Pokrovskiy O. On the Enhanced Accuracy of Kinetic Curve Building in Supercritical Fluid Extraction from Aroma Plants Using a New 3D-Printed Extract Collection Device. Molecules. 2020; 25(9):2008. https://doi.org/10.3390/molecules25092008
Chicago/Turabian StyleProkopchuk, Denis, and Oleg Pokrovskiy. 2020. "On the Enhanced Accuracy of Kinetic Curve Building in Supercritical Fluid Extraction from Aroma Plants Using a New 3D-Printed Extract Collection Device" Molecules 25, no. 9: 2008. https://doi.org/10.3390/molecules25092008
APA StyleProkopchuk, D., & Pokrovskiy, O. (2020). On the Enhanced Accuracy of Kinetic Curve Building in Supercritical Fluid Extraction from Aroma Plants Using a New 3D-Printed Extract Collection Device. Molecules, 25(9), 2008. https://doi.org/10.3390/molecules25092008