Phenolic Compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have Antioxidant and Anticancer Activity
Abstract
:1. Introduction
2. Results
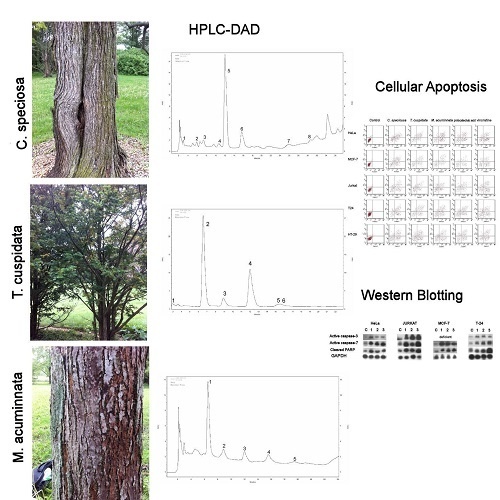
2.1. Targeted Profiling of Catechins and Phenols
2.1.1. C. speciosa
2.1.2. T. cuspidata
2.1.3. M. acuminata
2.2. Antioxidant Activities
2.3. Antiproliferative Activities
2.4. Apoptotic Cell Population
2.5. Detection of Caspase-3/7 Activity
2.6. Western Blotting Analyses of Caspases-3 and Caspase-7
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation and Cell Cultures
4.3. Analyses of Phenolic Compounds
4.4. Antioxidant Activity
4.5. Antiproliferative Activity
4.6. Apoptotic Cell Population
4.7. Caspase-Glo 3/7 Assay
4.8. Western Blotting of Caspase-3 and Caspase-7
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grace, O.M.; Prendergast, H.D.V.; Jager, A.K.; van Staden, J. Bark medicines used in traditional healthcare in KwaZulu-Natal, South Africa: An inventory. S. Afr. J. Bot. 2003, 69, 301–363. [Google Scholar] [CrossRef] [Green Version]
- Bello, I.; Shehu, M.W.; Musa, M.; Asmawi, M.Z.; Mahmud, R. Kigelia africana (Lam.) Benth. (Sausage tree): Phytochemistry and pharmacological review of a quintessential African traditional medicinal plant. J. Ethnopharmacol. 2016, 189, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, W.J.; Geldenhuys, C.J.; Esler, K.J. Response of Ocotea bullata, Curtisia dentata and Rapanea melanophloeos to medicinal bark stripping in the southern Cape, South Africa: Implications for sustainable use. South. For. 2012, 74, 183–193. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Chan, M.H.; Zong, Y.; Sheng, W.; He, Y.; Jiang, J.H.; Simonyi, A.; Gu, Z.; Fritsche, K.L.; Cui, J.; et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J. Neuroinflamm. 2013, 10, 15. [Google Scholar] [CrossRef]
- Juyal, D.; Thawani, V.; Thaledi, S.; Joshi, M. Ethnomedical properties of Taxus wallichiana zucc. (Himalayan yew). J. Tradit. Complement. Med. 2014, 4, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.X.; Yan, R.Y.; Liang, R.X.; Wang, W.; Yang, B. Bioactive polar compounds from stem bark of Magnolia officinalis. Fitoterapia 2012, 83, 356–361. [Google Scholar] [CrossRef]
- Hemmings, E.T.; Core, E.L. Archeological Evidence for Range Extension of the Catawba Tree (Catalpa speciosa Warder) in West Virginia. Castanea 1976, 41, 9–11. [Google Scholar]
- Xu, H.; Hu, G.; Dong, J.; Wei, Q.; Shao, H.; Lei, M. Antioxidative Activities and Active Compounds of Extracts from Catalpa Plant Leaves. Sci. World J. 2014, 2014, 857982. [Google Scholar] [CrossRef]
- Dvorska, M.; Zemlicka, M.; Muselik, J.; Karafiatova, J.; Suchy, V. Antioxidant activity of Catalpa bignonioides. Fitoterapia 2007, 78, 437–439. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and Healthcare. European Pharmacopoeia 9.0; Council of Europe: Strasbourg, France, 2017. [Google Scholar]
- Park, J.; Lee, J.; Jung, E.S.; Park, Y.; Kim, K.; Park, B.; Jung, K.S.; Park, E.; Kim, J.; Park, D. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur. J. Pharmacol. 2004, 496, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Farjon, A. World Checklist and Bibliography of Conifers. In World Checklists and Bibliographies, 3, 2nd ed.; Royal Botanic Gardens, Kew: Kew, UK, 2001; p. 300. [Google Scholar]
- Kawamura, F.; Kikuchi, Y.; Ohira, T.; Yatagai, M. Accelerated solvent extraction of paclitaxel and related compounds from the bark of Taxus cuspidata. J. Nat. Prod. 1999, 62, 244–247. [Google Scholar] [CrossRef]
- Kawamura, F.; Kikuchi, Y.; Ohira, T.; Yatagai, M. Phenolic constituents of Taxus cuspidata I: Lignans from the roots. J. Wood Sci. 2000, 46, 167–171. [Google Scholar] [CrossRef]
- Shen, C.C.; Ni, C.L.; Shen, Y.C.; Huang, Y.L.; Kuo, C.H.; Wu, T.S.; Chen, C.C. Phenolic constituents from the stem bark of Magnolia officinalis. J. Nat. Prod. 2009, 72, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparlo, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chem. 2013, 136, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Gadde, U.D.; Bravo, D.; Lillehoj, E.P.; Lillehoj, H.S. Growth-Promoting and Antioxidant Effects of Magnolia Bark Extract in Chickens Uninfected or Co-Infected with Clostridium perfringens and Eimeria maxima as an Experimental Model of Necrotic Enteritis. Curr. Dev. Nutr. 2018, 2, nzy009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, S.; Liang, Y.; Mu, Y.; Yan, H.; Liu, Q.; Geng, Y.; Wang, X.; Zhao, H. Rapid purification of antioxidants from Magnolia officinalis by semi-prep-HPLC with a two-step separation strategy guided by on-line HPLC-radical scavenging detection. J. Chromatogr. B 2018, 1100–1101, 140–147. [Google Scholar] [CrossRef]
- Huang, H.C.; Hsieh, W.Y.; Niu, Y.L.; Chang, T.M. Inhibition of melanogenesis and antioxidant properties of Magnolia grandiflora L. flower extract. BMC Complement. Altern. Med. 2012, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dong, W.; Zhang, D.; Jin, L.; Zhang, D.; Zhang, J.; Tao, S. Screening of Natural Antioxidants and Application In the Course of Perry Brewing. Energy Procedia 2012, 17, 1811–1816. [Google Scholar] [CrossRef] [Green Version]
- Veselova, M.V.; Fedoreev, S.A.; Vasilevskaya, N.A.; Denisenko, V.A.; Gerasimenko, A.V. From leaf to flower: Revisiting Goethe’s concepts on the “metamorphosis” of plants. Pharm. Chem. J. 2007, 41, 88–93. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid.-Based Complement. Altern. 2015, 2015, 593902. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.J.; Hu, S.Q.; Zhou, Z.X.; Liu, X.R.; Tang, J.B.; Shen, Y.Q. Dendrimers with the protocatechuic acid building block for anticancer drug delivery. J. Mater. Chem. B 2016, 4, 5236–5245. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, Y.; Lu, B.M.; Liu, Y.X.; Li, J.; Bao, J.K. Green tea catechins: A fresh flavor to anticancer therapy. Apoptosis 2014, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Arulvasu, C.; Sellamuthu, S.; Dinesh, D.; Prabhu, D.; Babu, G.; Vaseeharan, B.; Prabhu, N.M. Synergistic anticancer activity of curcumin and catechin: An in vitro study using human cancer cell lines. Microsc. Res. Tech. 2012, 75, 112–116. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef] [Green Version]
- Takanashi, K.; Suda, M.; Matsumoto, K.; Toda, C.I.K.; Toda, K.; Kawaguchi, K.; Senga, S.; Kobayashi, N.; Ichikawa, M.; Katoh, M.; et al. Epicatechin oligomers longer than trimers have anti-cancer activities, but not the catechin counterparts. Sci. Rep. 2017, 7, 7791. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A Novel Natural Agent for Cancer Prevention and Therapy. Curr. Mol. Med. 2012, 12, 1244–1252. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, L.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evid.-Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef]
- Shang, W.; Qiao, J.; Gu, C.; Yin, W.; Du, J.; Wang, W.; Zhu, M.; Han, M.; Lu, W. Anticancer activity of an extract from needles and twigs of Taxus cuspidata and its synergistic effect as a cocktail with 5-fluorouracil. BMC Complement. Altern. Med. 2011, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Marin, G.H.; Mansilla, E. Apoptosis induced by Magnolia Grandi fl ora extract in chlorambucil-resistant B-chronic lymphocytic leukemia cells. J. Cancer Res. Ther. 2010, 6, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wu, Q.; Yang, S.H. Ferulic acid promoting apoptosis in human osteosarcoma cell lines. Pak. J. Med. Sci. 2017, 33, 127–131. [Google Scholar] [CrossRef]
- Yin, M.C.; Lin, C.C.; Wu, H.C.; Tsao, S.M.; Hsu, C.K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef]
- Alvarado-Sansininea, J.J.; Sanchez-Sanchez, L.; Lopez-Munoz, H.; Escobar, M.L.; Flores-Guzman, F.; Tavera-Hernandez, R.; Jimenez-Estrada, M. Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules 2018, 23, 2579. [Google Scholar] [CrossRef]
- Bell, R.A.V.; Megeney, L.A. Evolution of caspase-mediated cell death and differentiation: Twins separated at birth. Cell Death Differ. 2017, 24, 1359–1368. [Google Scholar] [CrossRef]
- Turner, C.; Devitt, A.; Parker, K.; MacFarlane, M.; Giuliano, M.; Cohen, G.M.; Gregory, C.D. Macrophage-mediated clearance of cells undergoing caspase-3-independent death. Cell Death Differ. 2003, 10, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagawa, S.; Gu, J.; Honda, T.; McDonnell, T.J.; Swisher, S.G.; Roth, J.A.; Fang, B. Deficiency of Caspase-3 in MCF7 Cells Blocks Bax-mediated Nuclear Fragmentation but not Cell Death. Clin. Cancer Res. 2001, 7, 1474–1480. [Google Scholar]
- Sung, M.H.; Kwon, O.K.; Oh, S.R.; Lee, J.; Park, S.H.; Han, S.B.; Ahn, K.S. Azorella compacta methanolic extract induces apoptosis via activation of mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6821–6828. [Google Scholar] [CrossRef]
- Wu, B.T.; Hung, P.F.; Chen, H.C.; Huang, R.N.; Chang, H.H.; Kao, Y.H. The apoptotic effect of green tea (-)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the Cdk2 pathway. J. Agric. Food Chem 2005, 53, 5695–5701. [Google Scholar] [CrossRef]
- Spencer, J.P.; Schroeter, H.; Kuhnle, G.; Srai, S.K.; Tyrrell, R.M.; Hahn, U.; Rice-Evans, C. Epicatechin and its in vivo metabolite, 3’-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem. J. 2001, 354, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Huttemann, M.; Zielske, S.P. Epicatechin stimulates mitochondrial activity and selectively sensitizes cancer cells to radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef]
- Yang, J.S.; Liu, C.W.; Ma, Y.S.; Weng, S.W.; Tang, N.Y.; Wu, S.H.; Ji, B.C.; Ma, C.Y.; Ko, Y.C.; Funayama, S.; et al. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase- and mitochondria-dependent pathways. In Vivo 2012, 26, 971–978. [Google Scholar]
- Ji, B.C.; Hsu, W.H.; Yang, J.S.; Hsia, T.C.; Lu, C.C.; Chiang, J.H.; Yang, J.L.; Lin, C.H.; Lin, J.J.; Suen, L.J.; et al. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J. Agric. Food Chem. 2009, 57, 7596–7604. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krosniak, A.; Krosniak, M.; Marzec-Wroblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A-melanocarpa, A-arbutifolia, and A. xprunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Ellnain-Wojtaszek, M.; Zgorka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L-leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sulkowska-Ziaja, K.; Maslanka, A.; Szewczyk, A.; Muszynska, B. Physiologically Active Compounds in Four Species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, H.; Szewczyk, A.; Fugas, E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. under different light conditions. Plant Cell Tissue Org. 2012, 110, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yessoufou, K.; El-Settawy, A.A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.N.; Wang, J.Y.; Yu, L.L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef]
- Komina, A.; Palkina, N.; Aksenenko, M.; Tsyrenzhapova, S.; Ruksha, T. Antiproliferative and Pro-Apoptotic Effects of MiR-4286 Inhibition in Melanoma Cells. PLoS ONE 2016, 11, e0168229. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Species | Chemical Compound | Amount [mg 100g−1] D.W. |
|---|---|---|
| Catalpa speciosa | Caffeic acid | 3.04 ± 0.45 |
| p-Coumaric acid | 3.28 ± 0.44 | |
| Ferulic acid | 22.7 ± 0.18 | |
| Gallic acid | 1.57 ± 0.04 | |
| p-Hydroxybenzoic acid | 6.42 ± 0.03 | |
| Protocatechuic acid | 3.22 ± 0.02 | |
| Vanillic acid | 5.77 ± 0.22 | |
| Taxus cuspidata | Caffeic acid | 3.05 ± 0.01 |
| Chlorogenic acid | 8.30 ± 0.22 | |
| Gallic acid | 2.04 ± 0.07 | |
| p-Hydroxybenzoic acid | 2.42 ± 0.16 | |
| Hydroxycaffeic acid | 23.98 ± 1.3 | |
| Protocatechuic acid | 20.97 ± 0.56 | |
| Magnolia acuminnata | Ellagic acid | 0.43 ± 0.08 |
| Protocatechuic acid | 15.31 ± 1.19 |
| Species | Chemical Compound | Amount [mg 100g−1] D.W. |
|---|---|---|
| Catalpa speciosa | Catechin | 1.19 ± 0.05 |
| Magnolia acuminnata | Catechin | 85.47 ± 1.30 |
| Epicatechin | 22.78 ± 0.53 | |
| Epigallocatechin gallate | 14.22 ± 0.95 |
| Plant/Standard | DPPH Free Radical Scavenging Activity (IC50, µg mL−1) | β-Carotene-Linoleic Acid Assay (IC50, µg mL−1) |
|---|---|---|
| Catalpa speciosa | 4.4 ± 0.1a | 5.1 ± 0.1a |
| Taxus cuspidata | 4.2 ± 0.1b | 4.8 ± 0.1b |
| Magnolia acuminnata | 3.1 ± 0.1c | 3.6 ± 0.1c |
| BHT | 2.9 ± 0.1c | 3.2 ± 0.1c |
| Plant/Standard | HeLa | MCF-7 | Jurkat | T24 | HT-29 | HEK-293 |
|---|---|---|---|---|---|---|
| Catalpa speciosa | 58.3 ± 1.7 | 41.19 ± 1.6 | 41.4 ± 1.1 | 249.5 ± 2.9 | 111.5 ± 2.9 | ˃400 |
| Taxus cuspidata | 54.5 ± 1.9 | 39.51 ± 0.9 | 37.3 ± 0.5 | 220.1 ± 2.9 | 102.2 ± 3.1 | ˃400 |
| Magnolia acuminnata | 28.4 ± 1.3 | 16.20 ± 1.1 | 25.1 ± 1.1 | 152.8 ± 2.9 | 89.2 ± 2.5 | ˃400 |
| Catechin | 36.48 ± 1.2 | 17.64 ± 1.8 | 38.16 ± 0.7 | 183.28 ± 4.3 | 96.16 ± 1.5 | ˃400 |
| Protocatechuic acid | 39.10 ± 2.3 | 18.97 ± 2.1 | 47.35 ± 2.1 | 176.35 ± 2.1 | 95.35 ± 3.3 | ˃400 |
| Ferulic acid | 51.73 ± 3.5 | 43.85 ± 1.3 | 39.11 ± 2.3 | 227.26 ± 5.1 | 126.26 ± 4.4 | ˃400 |
| Hydroxycaffeic acid | 65.31 ± 2.4 | 58.11 ± 0.9 | 63.09 ± 1.4 | 176.12 ± 3.6 | 113.15 ± 2.9 | ˃400 |
| Vinblastine sulfate | 2.7 ± 0.06 | ‒ | 0.1 ± 0.09 | 65.7 ± 2.1 | 21.0 ± 0.1 | 50.1± 2.3 |
| Vincristine | 8.5 ± 0.1 | 4.63 ± 1.8 | 0.4 ± 0.05 | 89.8 ± 2.5 | 47.3 ± 0.2 | 78.3 ± 1.6 |
| Taxol | ‒ | 0.09 ± 0.009 | ‒ | ‒ | ‒ | ‒ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elansary, H.O.; Szopa, A.; Kubica, P.; A. Al-Mana, F.; Mahmoud, E.A.; Zin El-Abedin, T.K.A.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. https://doi.org/10.3390/molecules24030412
Elansary HO, Szopa A, Kubica P, A. Al-Mana F, Mahmoud EA, Zin El-Abedin TKA, Mattar MA, Ekiert H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules. 2019; 24(3):412. https://doi.org/10.3390/molecules24030412
Chicago/Turabian StyleElansary, Hosam O., Agnieszka Szopa, Paweł Kubica, Fahed A. Al-Mana, Eman A. Mahmoud, Tarek K. Ali Zin El-Abedin, Mohamed A. Mattar, and Halina Ekiert. 2019. "Phenolic Compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have Antioxidant and Anticancer Activity" Molecules 24, no. 3: 412. https://doi.org/10.3390/molecules24030412
APA StyleElansary, H. O., Szopa, A., Kubica, P., A. Al-Mana, F., Mahmoud, E. A., Zin El-Abedin, T. K. A., Mattar, M. A., & Ekiert, H. (2019). Phenolic Compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules, 24(3), 412. https://doi.org/10.3390/molecules24030412