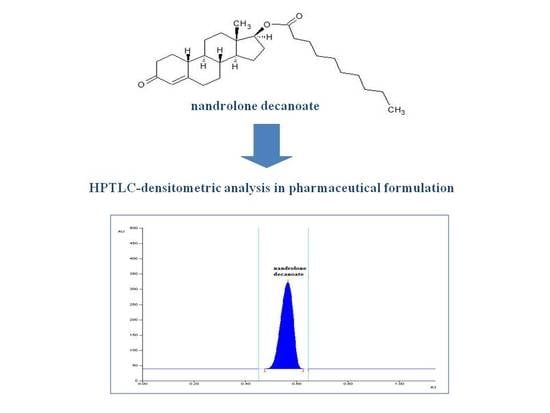
Simple and Accurate HPTLC-Densitometric Method for Assay of Nandrolone Decanoate in Pharmaceutical Formulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Validation Results
2.2.1. Specificity
2.2.2. Linearity
2.2.3. Limits of Detection and Quantification
2.2.4. Accuracy
2.2.5. Precision
2.2.6. Robustness
2.2.7. Analysis of Pharmaceutical Formulation
3. Materials and Methods
3.1. Reagents and Apparatus
3.1.1. Pure Standard
3.1.2. Sample
3.1.3. Solvents
3.1.4. Chromatographic Plates
3.1.5. Apparatus
3.1.6. Standard Solution
3.1.7. Sample Preparation
3.2. Method Validation
3.2.1. Specificity
3.2.2. Linearity
3.2.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.2.4. Precision
3.2.5. Accuracy
3.2.6. Robustness
3.3. Analysis of Pharmaceutical Formulation of Nandrolone Decanoate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zejc, A.; Gorczyca, M. Drug Chemistry (in Polish); PZWL: Warsaw, Poland, 2004. [Google Scholar]
- Buczko, W.; Danysz, A. Compendium of Pharmacology and Pharmacotherapy (in Polish); Edra Urban & Partner: Wrocław, Poland, 2016. [Google Scholar]
- Ozer, D.; Temizer, A. The determination of nandrolone and its metabolites in urine by gas chromatography-mass spectrometry. Eur. J. Drug. Metab. Pharmacokinet. 1997, 22, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Segura, J.; Ventura, R. Quantitation of 17- beta-nandrolone metabolites in boar and horse urine by gas chromatography-mass spectrometry. Anal. Chim. Acta. 2007, 14, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Zhang, L.; Li, G.L.; Zhang, H.T.; Yang, X.F.; Liu, J.W.; Li, R.F.; Wang, Z.L.; Wang, J.H. Analysis of 19-nortestosterone residue in animal tissues by ion-trap gas chromatography-tandem mass spectrometry. J. Zhejiang Univ. Sci. B. 2011, 12, 460–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, J.; Das, A.; Chakrabarty, U.S.; Sahoo, B.K.; Dey, G.; Choudhury, H.; Pal, T.K. Development and validation of RPHPLC method to determine nandrolone phenylpropionate in different pharmaceutical formulations. Acta Pol. Pharm.–Drug Res. 2011, 68, 155–160. [Google Scholar]
- Dołowy, M.; Pyka, A. TLC-densitometric method for qualitative analysis of betamethasone and its related compounds in pharmaceutical preparations. Acta Pol. Pharm.-Drug Res. 2014, 71, 922–932. [Google Scholar]
- Dołowy, M.; Pyka-Pająk, A.; Filip, K.; Zagrodzka, J. A validated TLC-densitometric method for the determination of mesterolone in bulk material and in tablets. BioMed. Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dołowy, M.; Pyka-Pająk, A. Comparison of the limits of detection and quantification of estradiol hemihydrate determined by thin-layer chromatography using different chromatographic conditions. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 264–270. [Google Scholar] [CrossRef]
- Dołowy, M.; Kozik, V.; Bąk, A.; Jampilek, J.; Barbusiński, K.; Thomas, M.; Pyka-Pająk, A. Rapid and simple TLC-densitometric method for assay of clobetasol propionate in topical solution. Molecules 2017, 22, 1888–1900. [Google Scholar]
- Dołowy, M.; Piontek, A.; Pyka-Pająk, A. Validated RP-TLC method with densitometry for assay of finasteride in simple pharmaceutical dosage form. Curr. Pharmaceut. Anal. 2018, 14, 298–305. [Google Scholar]
- Bhawani, S.A.; Sulaiman, O.; Hashim, R.; Ibrahim, M.N. Thin-layer chromatographic analysis of steroids: A review. Trop. J. Pharm. Res. 2010, 9, 301–313. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Gulzar, U. Effective separation and simultaneous analysis of anabolic androgenic steroids (AAS) in their pharmaceutical formulations by a validated TLC-densitometry method. Chem. Cent. J. 2012, 6, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.D.; Sharma, A.K. Thin layer chromatographic study of some anabolic sport steroids. Indian Journ. Forensic Sci. 1991, 5, 117–122. [Google Scholar]
- Lekic, M.; Korac, F.; Sober, M.; Marjanovic, A. Planar chromatography of steroid hormones and anabolics. Acta Chim. Slov. 2007, 54, 88–91. [Google Scholar]
- Bagocsi, B.; Fabian, D.; Lauko, A.; Mezei, M.; Maho, S.; Vegh, Z.; Ferenczi-Fodor, K. Comparison of OPLC and other chromatographic methods (TLC, HPLC, and GC) for in-process purity testing of nandrolone. J. Planar Chromatogr. 2002, 15, 252–257. [Google Scholar] [CrossRef]
- Bagocsi, B.; Vegh, Z.; Ferenczi-Fodor, K. Optimization of the visualization of steroids separated by OPLC. J. Planar Chromatogr. 2008, 21, 107–112. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2(R1), ICH. 2005. Available online: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed on 10 October 2018).
- Polish Pharmacopoeia X.; Polish Pharmaceutical Society: Warsaw, Poland, 2014.
Sample Availability: Samples of the compounds are not available from the authors. |




| Parameter | Results |
|---|---|
| Wavelength (nm) | 245 |
| Linearity range (μg/spot) | 0.780–12.500 |
| Slope | 2155.5 |
| Standard deviation of slope | 21.8 |
| Coefficient of variation (%CV) of slope | 1.0 |
| Intercept | 535.1 |
| Standard deviation of intercept | 150.8 |
| Correlation coefficient | 0.9998 |
| Standard deviation of residuals | 222.9 |
| F | 9838.1 |
| Significance level | <0.0001 |
| Initial Amount of Nandrolone Decanoate in Drug Sample (μg/μL) | % Amount Nandrolone Decanoate Standard Added | Theoretical Total Amount of Nandrolone Decanoate in Drug Sample (μg/spot) | Average Amount of Nandrolone Decanoate Recovered (μg/spot) ±SD | Recovery R (%) | Average Recovery R (%) |
|---|---|---|---|---|---|
| 0.50 | 50 | 3.75 | 3.76 ± 0.21 | 100.3 | 99.0 |
| 0.50 | 100 | 5.00 | 4.93 ± 0.10 | 98.6 | |
| 0.50 | 150 | 6.25 | 6.14 ± 0.11 | 98.2 |
| Intra-Day Precision (Repeatability, n = 3) | ||
| Amount of Nandrolone Decanoate (μg/spot) | Measured Peak Area ± SD | %CV of Peak Area |
| 0.780 | 2139 ± 23 | 1.08 |
| 1.560 | 3857 ± 65 | 1.68 |
| 3.120 | 7370 ± 86 | 1.17 |
| Inter-Day Precision (Intermediate Precision, n = 3) | ||
| Amount of Nandrolone Decanoate (μg/spot) | Measured Peak Area ± SD | %CV of Peak Area |
| 0.780 | 2124 ± 17 | 0.80 |
| 1.560 | 3799 ± 38 | 0.97 |
| 3.120 | 7128 ± 84 | 1.18 |
| Variations of Chromatographic Conditions | %CV of Peak Area | % Assay of Nandrolone Decanoate |
|---|---|---|
| Volume of mobile phase (±5 mL) | 1.07 | 98.8 |
| Time of activation of chromatographic plates (±5 min) | 0.76 | 98.0 |
| Chamber saturation time (±5 min) | 0.63 | 98.4 |
| Development distance (±10 mm) | 1.20 | 100.1 |
| Volume of sample spotting onto chromatographic plate (±1 μL) | 1.42 | 99.3 |
| Parameter | Data |
|---|---|
| Number of analyses | 6 |
| Label claim of nandrolone decanoate (mg/mL) | 50.0 |
| Average amount of nandrolone decanoate (mg/mL) | 50.5 |
| Minimum amount of nandrolone decanoate (mg/mL) | 50.1 |
| Maximum amount of nandrolone decanoate (mg/mL) | 50.9 |
| Standard deviation (SD) | 0.4 |
| Coefficient of variation (%CV) | 0.8 |
| Confidence interval of arithmetic mean with confidence level equal 95% | µ = 50.5 ± 0.4 |
| Amount of nandrolone decanoate (%) in relation to label claim | 101.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dołowy, M.; Pyka-Pająk, A.; Jampílek, J. Simple and Accurate HPTLC-Densitometric Method for Assay of Nandrolone Decanoate in Pharmaceutical Formulation. Molecules 2019, 24, 435. https://doi.org/10.3390/molecules24030435
Dołowy M, Pyka-Pająk A, Jampílek J. Simple and Accurate HPTLC-Densitometric Method for Assay of Nandrolone Decanoate in Pharmaceutical Formulation. Molecules. 2019; 24(3):435. https://doi.org/10.3390/molecules24030435
Chicago/Turabian StyleDołowy, Małgorzata, Alina Pyka-Pająk, and Josef Jampílek. 2019. "Simple and Accurate HPTLC-Densitometric Method for Assay of Nandrolone Decanoate in Pharmaceutical Formulation" Molecules 24, no. 3: 435. https://doi.org/10.3390/molecules24030435
APA StyleDołowy, M., Pyka-Pająk, A., & Jampílek, J. (2019). Simple and Accurate HPTLC-Densitometric Method for Assay of Nandrolone Decanoate in Pharmaceutical Formulation. Molecules, 24(3), 435. https://doi.org/10.3390/molecules24030435