A Hybrid Material Combined Copper Oxide with Graphene for an Oxygen Reduction Reaction in an Alkaline Medium
Abstract
:1. Introduction
2. Results and Discussion
Structural Characterization
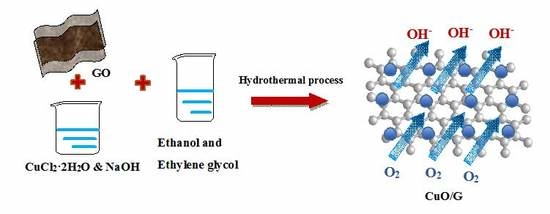
3. Experimental
Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, Y.; Xu, R. Nickel-based cocatalysts for photocatalytic hydrogen production. Appl. Surf. Sci. 2015, 351, 779–793. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ashokkumar, M.; Gullapalli, H.; Gong, Y.; Vajtai, R.; Thanikaivelan, P.; Ajayan, P.M. Nitrogen-rich carbon nano-onions for oxygen reduction reaction. Carbon 2018, 130, 645–651. [Google Scholar] [CrossRef]
- Grove, W.R.; Esq, M.A. XXIV. On voltaic series and the combination of gases by platinum. Philos. Mag. 1966, 14, 127–130. [Google Scholar]
- Nasini, U.B.; Gopal, V.; Bairi; Kumar, S.; Ramasahayam; Bourdo, S.E.; Viswanathan, T.; Shaikh, A.U. Oxygen Reduction Reaction Studies of Phosphorus and Nitrogen Co-Doped Mesoporous Carbon Synthesized via Microwave Technique. Chemelectrochem 2014, 1, 573–579. [Google Scholar] [CrossRef]
- Awad, M.I.; Ohsaka, T. An electrocatalytic oxygen reduction by copper nanoparticles-modified Au(100)-rich polycrystalline gold electrode in 0.5 M KOH. J. Power Sources 2013, 226, 306–312. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Shao, X.; Xu, H.Y.; Xie, M.; Deng, S.X.; Wang, H.; Liu, J.B.; Yan, H. Facile synthesis of porous LiMn2O4 spheres as cathode materials for high-power lithium ion batteries. J. Power Sources 2013, 226, 140–148. [Google Scholar] [CrossRef]
- Neburchilov, V.; Wang, H.; Martin, J.J.; Wei, Q. A review on air cathodes for zinc–air fuel cells. J. Power Sources 2010, 195, 1271–1291. [Google Scholar] [CrossRef]
- Bruce, P.G.; Hardwick, L.J.; Abraham, K.M. Lithium-air and lithium-sulfur batteries. Mrs. Bull. 2011, 36, 506–512. [Google Scholar] [CrossRef]
- Jia, Q.; Zhao, Z.; Cao, L.; Li, J.; Ghoshal, S.; Davies, V.; Stavitski, E.; Attenkofer, K.; Liu, Z.; Li, M.; et al. Roles of Mo Surface Dopants in Enhancing the ORR Performance of Octahedral PtNi Nanoparticles. Nano Lett. 2018, 18, 798–804. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P.; Hay, A.S. Catalyst Precursors, Catalysts and Methods of Producing Same. U.S. Patent 8580704B2, 12 December 2013. [Google Scholar]
- Xue, Y.; Yu, D.; Dai, L.; Wang, R.; Li, D.; Roy, A.; Lu, F.; Chen, H.; Liu, Y.; Qu, J. Three-dimensional B,N-doped graphene foam as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. PCCP 2013, 15, 12220–12226. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef]
- Takashima, T.; Hashimoto, K.; Nakamura, R. Inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J. Am. Chem. Soc. 2012, 134, 18153–18156. [Google Scholar] [CrossRef]
- Hossen, M.M.; Artyushkova, K.; Atanassov, P.; Serov, A. Synthesis and characterization of high performing Fe-N-C catalyst for oxygen reduction reaction (ORR) in Alkaline Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 214–221. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Hulicovajurcakova, D.; Qiao, S.Z. Enhanced electrochemical catalytic activity by copper oxide grown on nitrogen-doped reduced graphene oxide. J. Mater. Chem. A 2013, 1, 13179–13185. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Thorum, M.S. Electroreduction of dioxygen for fuel-cell applications: Materials and challenges. Inorg. Chem. 2010, 49, 3557–3566. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Wang, F.B.; Xia, X.H. Bioinspired copper catalyst effective for both reduction and evolution of oxygen. Nat. Commun. 2014, 5, 5285. [Google Scholar] [CrossRef] [Green Version]
- Koninck, M.D.; Poirier, S.C.; Marsan, B. CuxCo3-xO4 used as bifunctional electrocatalyst. J. Electrochem. Soc. 2006, 153, A2103–A2110. [Google Scholar] [CrossRef]
- Koninck, M.D.; Poirier, S.C.; Marsan, B. CuxCo3-xO4 Used as Bifunctional Electrocatalyst II. Electrochemical Characterization for the Oxygen Reduction Reaction. J. Electrochem. Soc. 2007, 154, A381–A388. [Google Scholar]
- Ania, C.O.; Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T.J. New copper/GO based material as an efficient oxygen reduction catalyst in an alkaline medium: The role of unique Cu/rGO architecture. Appl. Catal. B Environ. 2015, 163, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Blanford, C.F.; Heath, R.S.; Armstrong, F.A. A stable electrode for high-potential, electrocatalytic O(2) reduction based on rational attachment of a blue copper oxidase to a graphite surface. Chem. Commun. 2007, 2007, 1710–1712. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, W.-p.; Zhang, X.; Peng, W. CuO nanoparticles on sulfur-doped graphene for nonenzymatic glucose sensing. Electrochim Acta 2015, 156, 244–251. [Google Scholar] [CrossRef]
- Sherly, E.D.; Vijaya, J.J.; Kennedy, L.J. Visible-light-induced photocatalytic performances of ZnO-CuO nanocomposites for degradation of 2,4-dichlorophenol. Chin. J. Catal. 2015, 36, 1263–1272. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. The Raman Fingerprint of Graphene. 2006. [Google Scholar] [CrossRef]
- Bian, W.; Yang, Z.; Strasser, P.; Yang, R. A CoFe2O4/graphene nanohybrid as an efficient bi-functional electrocatalyst for oxygen reduction and oxygen evolution. J. Power Sources 2014, 250, 196–203. [Google Scholar] [CrossRef]
- Hong, Y.; Tian, C.; Jiang, B.; Wu, A.; Zhang, Q.; Tian, G.; Fu, H. Facile synthesis of sheet-like ZnO assembly composed of small ZnO particles for highly efficient photocatalysis. J. Mater. Chem. A 2013, 1, 5700–5708. [Google Scholar] [CrossRef]
- Li, S.S.; Lv, J.J.; Hu, Y.Y.; Zheng, J.N.; Chen, J.R.; Wang, A.J.; Feng, J.J. Facile synthesis of porous Pt-Pd nanospheres supported on reduced graphene oxide nanosheets for enhanced methanol electrooxidation. J. Power Sources 2014, 247, 213–218. [Google Scholar] [CrossRef]
- Li, S.S.; Wang, A.J.; Hu, Y.Y.; Fang, K.M.; Chen, J.R.; Feng, J.J. One-step, seedless wet-chemical synthesis of gold@palladium nanoflowers supported on reduced graphene oxide with enhanced electrocatalytic properties. J. Mater. Chem. A 2014, 2, 18177–18183. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Chen, W. Recent developments in copper-based, non-noble metal electrocatalysts for the oxygen reduction reaction. Chinese J. Catal. 2016, 37, 1049–1061. [Google Scholar] [CrossRef]
- Hung, T.F.; Bei, W.; Tsai, C.W.; Tu, M.H.; Wang, G.X.; Liu, R.S.; Tsai, D.P.; Lo, M.Y.; Shy, D.S.; Xing, X.K. Sulfonation of graphene nanosheet-supported platinum via a simple thermal-treatment toward its oxygen reduction activity in acid medium. Int. J. Hydrogen Energy 2012, 37, 14205–14210. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, H.; Zhou, B.; Huang, F.; Zhou, S.; Xiao, W.; Wang, D. Hierarchical MoS2–rGO nanosheets with high MoS2 loading with enhanced electro-catalytic performance. Appl. Surf. Sci. 2015, 358, 152–158. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. NiCoMnO4 nanoparticles on N-doped graphene: Highly efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions. Appl. Catal. B Environ. 2017, 201, 241–252. [Google Scholar] [CrossRef]
- Yan, Z.; Qi, H.; Bai, X.; Huang, K.; Chen, Y.-R.; Wang, Q. Mn doping of cobalt oxynitride coupled with N-rGO nanosheets hybrid as a highly efficient electrocatalyst for oxygen reduction and oxygen evolution reaction. Electrochim. Acta 2018, 283, 548–559. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, F. Self-assembled fabrication and flame-retardant properties of reduced graphene oxide/waterborne polyurethane nanocomposites. J. Therm. Anal. Calorim. 2014, 118, 1561–1568. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Sample | a | b | c |
|---|---|---|---|
| CuO | 0.469 | 0.343 | 0.513 |
| CuO/G | 0.469 | 0.342 | 0.513 |
| Sample | CuO | CuO/G |
|---|---|---|
| i0 (mA/cm2) | 2.12 × 10−8 | 3.79 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Huang, T.; Jiang, Z.; Sun, M.; Tang, C. A Hybrid Material Combined Copper Oxide with Graphene for an Oxygen Reduction Reaction in an Alkaline Medium. Molecules 2019, 24, 441. https://doi.org/10.3390/molecules24030441
Yu J, Huang T, Jiang Z, Sun M, Tang C. A Hybrid Material Combined Copper Oxide with Graphene for an Oxygen Reduction Reaction in an Alkaline Medium. Molecules. 2019; 24(3):441. https://doi.org/10.3390/molecules24030441
Chicago/Turabian StyleYu, Jiemei, Taizhong Huang, Zhankun Jiang, Min Sun, and Chengchun Tang. 2019. "A Hybrid Material Combined Copper Oxide with Graphene for an Oxygen Reduction Reaction in an Alkaline Medium" Molecules 24, no. 3: 441. https://doi.org/10.3390/molecules24030441
APA StyleYu, J., Huang, T., Jiang, Z., Sun, M., & Tang, C. (2019). A Hybrid Material Combined Copper Oxide with Graphene for an Oxygen Reduction Reaction in an Alkaline Medium. Molecules, 24(3), 441. https://doi.org/10.3390/molecules24030441