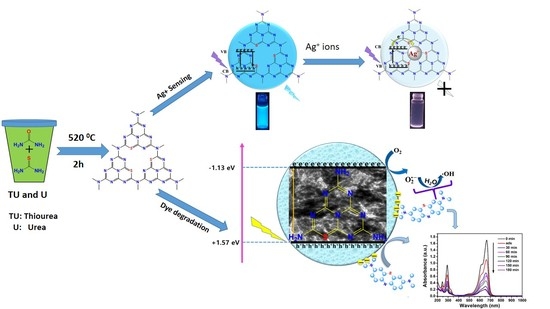
Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Characterization of SCNPNS
2.2. UV–Vis and Fluorescence Properties of SCNPNS
2.3. Selective Fluorescence Sensing of SCNPNS toward Ag+
2.4. Plausible Mechanism of PL Quenching of SCNPNS with Ag+ Ions
2.5. Photocatalytic Degradation of Dyes under Visible Light
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the SCNPNS
3.3. Instrumental Characterization
3.4. Fluorescence Detection of Ag+ Ions
3.5. Photocatalytic Activity of the SCNPNS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gavade, N.L.; Kadam, A.N.; Gaikwad, Y.B.; Dhanavade, M.J.; Garadkar, K.M. Decoration of biogenic AgNPs on template free ZnO nanorods for sunlight driven photocatalytic detoxification of dyes and inhibition of bacteria. J. Mater. Sci. Mater. Electron. 2016, 27, 11080–11091. [Google Scholar] [CrossRef]
- Afroze, S.; KantiSen, T. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Poll. 2018, 229, 225. (In press) [Google Scholar] [CrossRef]
- Ho, W.C.J.; Tay, Q.; Qi, H.; Huang, Z.; Li, J.; Chen, Z. Photocatalytic and adsorption performances of faceted cuprous oxide (Cu2O) particles for the removal of methyl orange (MO) from aqueous media. Molecules 2017, 22, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, D.; Roy, R.; Azzouz, A. Advances in catalytic oxidation of organic pollutants—Prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B Environ. 2015, 174–175, 277–292. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Kyung, H.; Lee, J.; Choi, W. Simultaneous and synergistic conversion of dyes and heavy metal ions in aqueous TiO2 suspensions under visible-light illumination. Environ. Sci. Technol. 2005, 39, 2376–2382. [Google Scholar] [CrossRef]
- Jiu, J.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The effect of light and humidity on the stability of silver nanowire transparent electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Xing, F.F.; He, S.J.; Song, S.P.; Wang, L.H.; Long, Y.T.; Li, D.; Fan, C.H. A graphene-based fluorescent nanoprobe for silver (I) ions detection by using graphene oxide and a silver-specific oligonucleotide. Chem. Commun. 2010, 46, 2596–2598. [Google Scholar] [CrossRef]
- Guo, Z.B.; Zheng, Y.F.; Xu, H.l.; Zheng, B.D.; Qiu, W.W.; Liu, G.D. Lateral flow test for visual detection of silver(I) based on cytosine-Ag(I)-cytosine interaction in C-rich oligonucleotides. Microchim. Acta 2017, 84, 243–250. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Liu, Y.; Xiao, Q. Red emission nitrogen, boron, sulfur co-doped carbon dots for “on-off-on” fluorescent mode detection of Ag+ ions and l-cysteine in complex biological fluids and living cells. Anal. Chim. Acta 2018, 1035, 192–202. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Y.; Lu, S.; Li, Y.; Liu, X.; Qin, Y.; Zheng, L. A highly selective TPE-based AIE fluorescent probe is developed for the detection of Ag+. RSC Adv. 2018, 8, 19701–19706. [Google Scholar] [CrossRef]
- Manzoori, J.L.; Abdolmohammad-Zadeh, H.; Amjadi, M. Ultra-trace determination of silver in water samples by electrothermal atomic absorption spectrometry after preconcentration with a ligand-less cloud point extraction methodology. J. Hazard Mater. 2007, 144, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanesh, N.; Suresh, S.; Prabhu, J.; Kannan, K.; Kannan, V.R.; Nandhakumar, R. Ratiometric fluorescent chemosensor for silver ion and its bacterial cell imaging. Opt. Mater. 2018, 82, 123–129. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Z.; Wang, M.; Yang, Z.; Ran, X.; He, W. A new BODIPY-derived ratiometric senor with internal charge transfer (ICT) effect: Colorimetric/fluorometric sensing of Ag+. Dalton Trans. 2018, 47, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zhu, C.Q. Functionalized cadmium sulfide quantum dots as fluorescence probe for silver ion determination. Anal. Chim. Acta 2005, 546, 147–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+ and Hg2+. Anal. Chim. Acta 2015, 870, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, Z.; Lin, F.; Guo, F.; Alamry, K.A.; Taib, L.A.; Asiri, A.M.; Wang, X. Sulfur-Doped Carbon Nitride Polymers for Photocatalytic Degradation of Organic Pollutant and Reduction of Cr(VI). Molecules 2017, 22, 572–589. [Google Scholar] [CrossRef]
- Wang, Y.; Hai, X.; Shuang, E.; Chen, M.; Yang, T.; Wang, J. Boronic acid functionalized g-C3N4 nanosheets for ultrasensitive and selective sensing of glycoprotein in the physiological environment. Nanoscale 2018, 10, 4913–4920. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.B.; Vázquez, A.; Martinez, D.S.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Kadam, A.N.; Bhopate, D.P.; Kondalkar, V.V.; Majhi, S.M.; Bathula, C.D.; Tran, A.V.; Lee, S.W. Facile synthesis of Ag-ZnO core–shell nanostructures with enhanced photocatalytic activity. J. Ind. Eng. Chem. 2018, 61, 78–86. [Google Scholar] [CrossRef]
- Nipane, S.V.; Lee, S.W.; Gokavi, G.S.; Kadam, A.N. In situ one pot synthesis of nanoscale TiO2-anchored reduced graphene oxide (RGO) for improved photodegradation of 5-fluorouracil drug. J. Mater. Sci: Mater Electron 2018, 29, 16553–16564. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.H.; Zhong, H.R.; Qiu, S.W.; Liang, Y.W.; Zhuang, X.Y.; Singh, A.; Kumar, A. Fluorescence sensing and photocatalytic properties of a 2D stable and biocompatible Zn(II)-based polymer. J. Mol. Struct. 2018, 1158, 264–270. [Google Scholar] [CrossRef]
- Fang, L.J.; Wang, X.L.; Zha, J.J.; Li, Y.H.; Wang, Y.L.; Du, X.L.; He, Z.F.; Zeng, H.D.; Yang, H.G. One-step fabrication of porous oxygen-doped g-C3N4 with feeble nitrogen vacancies for enhanced photocatalytic performance. Chem. Commun. 2016, 52, 4408–4411. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Chen, G.; Li, C.; Han, Z.; Hu, Y.; Meng, Q. Doping effect of non-metal group in porous ultrathin g-C3N4 nanosheets towards synergistically improved photocatalytic hydrogen evolution. Nanoscale 2018, 10, 5239–5245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT Study on sulfur-doped g-C3N4 nanosheets as a photocatalyst for CO2 reduction reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable synthesis of mesoporous sulfur-doped carbon nitride materials for enhanced visible light photocatalytic degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L.; Li, Y. Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts. Mater. Res. Bull. 2013, 48, 3919–3925. [Google Scholar] [CrossRef]
- Tanga, L.; Feng, C.; Deng, Y.; Zeng, G.; Wang, J.; Liu, Y.; Feng, H.; Wang, J. Enhanced photocatalytic activity of ternary Ag/g-C3N4/NaTaO3 photocatalysts under wide spectrum light radiation: The high potential band protection mechanism. Appl. Catal. B Environ. 2018, 230, 102–114. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Liu, C.; Dong, F.; Zhang, T.; Du, X.; Yu, S.; Zhang, Y. In situ co-pyrolysis fabrication of CeO2/g-C3N4 n–n type heterojunction for synchronously promoting photo-induced oxidation and reduction properties. J. Mater. Chem. A. 2015, 3, 17120–17129. [Google Scholar] [CrossRef]
- Horvath, B.E.; Kroke, E.; Svoboda, I.; Fuess, H.; Riedel, R. Potassium melonate, K3[C6N7(NCN)3]·5H2O, and its potential use for the synthesis of graphite-like C3N4 materials. New. J. Chem. 2005, 29, 693–699. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, D.H.; Lu, D. Nanonization of g-C3N4 with the assistance of activated carbon for improved visible light photocatalysis. RSC Adv. 2019, 6, 66814–66821. [Google Scholar] [CrossRef]
- Hong, L.X.; Zheng-Xin, T.; Xian-Zhou, Z. Molecular structure, IR spectra of 2-mercaptobenzothiazole and 2-mercaptobenzoxazole by density functional theory and ab initio Hartree–Fock calculations. Spectrochim. Acta A 2009, 74, 168–173. [Google Scholar]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.; Sun, J. Enhanced photocatalytic activity over the Ag2O–g-C3N4 composite under visible light. Catal. Sci. Technol. 2014, 4, 758–765. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Mesoporous Phosphorus-Doped g C3N4 Nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS. Appl. Mater. Inter. 2015, 7, 16850–16856. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sensor. Actuat. B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.X.; Yin, S.; Xu, H.; Xu, L.; Xu, Y.G. Preparation of sphere-like g-C3N4/BiOI photocatalysts via a reactable ionic liquid for visible-light-driven photocatalytic degradation of pollutants. J. Mater. Chem. A 2014, 2, 5340–5351. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. Appl. Catal. B Environ. 2017, 203, 300–313. [Google Scholar] [CrossRef]
- Lan, Z.A.; Zhang, G.; Wang, X. A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting. Appl. Catal. B Environ. 2016, 192, 116–125. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Q.; Yan, X.; Yang, M.; Ye, R.; Du, D.; Lin, Y. “On-Off-On” fluorescence sensor based on g-C3N4 nanosheets for selective and sequential detection of Ag+ and S2−. Talanta 2017, 168, 168–173. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357–5366. [Google Scholar] [CrossRef]
- Pankaj, A.; Tewari, K.; Singh, S.; Singh, P.S. Waste candle soot derived nitrogen doped carbon dots based fluorescent sensor probe: An efficient and inexpensive route to determine Hg (II) and Fe(III) from water. J. Environ. Chem. Engin. 2018, 6, 5561–5569. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Nitrogen- doped graphene quantum dots: “Turn-off” fluorescent probe for detection of Ag+ ions. J. Fluoresc. 2016, 26, 297–305. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Hwang, S.K.; Park, K.Y.; Kim, D.B.; Chang, S.K. Fluorescence sensing of Ag+ ions by desulfurization of an acetylthiourea derivative of 2-(2-hydroxyphenyl)benzothiazole. Dyes Pigments 2017, 147, 413–419. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large scale synthesis of graphene quantum dots (GQDs) from waste biomass and their use as an efficient and selective photoluminescence on–off–on probe for Ag+ ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, W.; Wang, S.; Peng, H.; Hu, X.; Ying, Y. Monolayer g-C3N4 fluorescent sensor for sensitive and selective colorimetric detection of silver ion from aqueous Samples. J. Fluoresc. 2016, 26, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, H.; Shao, G.; Gan, F. A tetraphenylethylene-based “turn on” fluorescent sensor for the rapid detection of Ag+ ions with high selectivity. J. Photoch. Photobio. A 2015, 301, 14–19. [Google Scholar] [CrossRef]
- Chen, D.; Li, S.; Zheng, F. Water soluble sulfur quantum dots for selective Ag+ sensing based on the ion aggregation-induced photoluminescence enhancement. Anal. Method 2016, 8, 632–636. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, T.Y.; Li, H.W.; Liu, Z.; Wu, Y. Microwave-assisted synthesis of BSA-protected small gold nanoclusters and their fluorescence-enhanced sensing of silver(I) ion. Nanoscale 2012, 4, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.Y.; Qi, H.; Zhang, Q.F.; Chen, J.; Wang, Y.; Wang, B.; Wang, S.J.; Yu, C. Detection of silver(I) ions based on the controlled self-assembly of a perylene fluorescence probe. Anal. Biochem. 2012, 30, 48–52. [Google Scholar] [CrossRef]
- Bian, W.; Zhang, H.; Yu, Q.; Shi, M.; Shuang, S.; Cai, Z.; Choi, M.F. Detection of Ag+ using graphite carbon nitride nanosheets based on fluorescence quenching. Spectrochim. Acta A 2016, 169, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.G.; Dige, N.C.; Desai, N.K.; Patil, S.R.; Kondalkar, V.V.; Hong, S.K.; HwanLee, K. Selective detection of Co2+ by fluorescent nano probe: Diagnostic approach for analysis of environmental samples and biological activities. Spectrochim. Acta A 2018, 198, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheet: A highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Anal. Chem. 2013, 85, 5595–5599. [Google Scholar] [CrossRef]
- Gavade, N.L.; Babar, S.B.; Kadam, A.N.; Gophane, A.D.; Garadkar, K.M. Fabrication of M@CuxO/ZnO (M= Ag, Au) heterostructured nanocomposite with enhanced photocatalytic performance under sunlight. Ind. Eng. Chem. 2017, 56, 14489–14501. [Google Scholar] [CrossRef]
- Babu, B.; Kadam, A.N.; Rao, G.T.; Lee, S.W.; Byon, C.; Shim, J. Enhancement of visible-light-driven photoresponse of Mn-doped SnO2 quantum dots obtained by rapid and energy efficient synthesis. J. Lumin. 2018, 195, 283–289. [Google Scholar] [CrossRef]
- Liu, X.; Jin, A.; Jia, Y.; Xia, T.; Deng, C.; Zhu, M.; Chen, C.; Chen, X. Synergy of adsorption and visible-light photocatalytic degradation of methylene blue by a bifunctional Z-scheme heterojunction of WO3/g-C3N4. Appl. Surf. Sci. 2017, 405, 359–371. [Google Scholar] [CrossRef]
- Xie, M.; Wei, W.; Jiang, Z.; Xu, Y.; Xie, J. Carbon nitride nanowires/nanofibers: A novel template-free synthesis from a cyanuric chloride-melamine precursor towards enhanced adsorption and visible-light photocatalytic performance. Ceram. Int. 2016, 42, 4158–4170. [Google Scholar] [CrossRef]
- Mao, C.; Zhao, Y.; Qiu, X.; Zhu, J.; Burda, C. Synthesis, characterization and computational study of nitrogen-doped CeO2 nanoparticles with visible-light activity. Phys. Chem. Chem. Phys. 2008, 10, 5633–5638. [Google Scholar] [CrossRef]
- Han, X.; Yao, C.; Yuan, A.; Xi, F.; Dong, X.; Liu, J. Enhanced charge separation ability and visible light photocatalytic performance of graphitic carbon nitride by binary S. , B co-doping. Mater. Res. Bull. 2018, 107, 477–483. [Google Scholar] [CrossRef]
- Kishore, K.; Guha, S.N.; Mahadevan, J.; Moorthy, P.N.; Mittal, J.P. Redox reactions of methylene blue: A pulse radiolysis study. Int. J. Radiat. Appl. Instrum. Radiat. Phys. Chem. 1989, 34, 721–727. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Fluorescent Probe | Limit of Detection (LOD, μM) | Ref. |
|---|---|---|
| Nitrogen-doped GQDs | 0.168 | [48] |
| CQDs | 0.5 | [49] |
| 2-(2-Hydroxyphenyl) benzothiazole dye | 0.76 | [50] |
| GQDs | 300 | [51] |
| Monolayer g-C3N4 | 0.0523 | [52] |
| Tetraphenylethylene | 0.874 | [53] |
| S-QDs | 0.810 | [54] |
| BSA protected small gold nanoclusters | 1 | [55] |
| SCNPNS | 0.057 | Present work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadam, A.N.; Moniruzzaman, M.; Lee, S.-W. Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes. Molecules 2019, 24, 450. https://doi.org/10.3390/molecules24030450
Kadam AN, Moniruzzaman M, Lee S-W. Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes. Molecules. 2019; 24(3):450. https://doi.org/10.3390/molecules24030450
Chicago/Turabian StyleKadam, Abhijit N., Md. Moniruzzaman, and Sang-Wha Lee. 2019. "Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes" Molecules 24, no. 3: 450. https://doi.org/10.3390/molecules24030450
APA StyleKadam, A. N., Moniruzzaman, M., & Lee, S. -W. (2019). Dual Functional S-Doped g-C3N4 Pinhole Porous Nanosheets for Selective Fluorescence Sensing of Ag+ and Visible-Light Photocatalysis of Dyes. Molecules, 24(3), 450. https://doi.org/10.3390/molecules24030450