Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers
Abstract
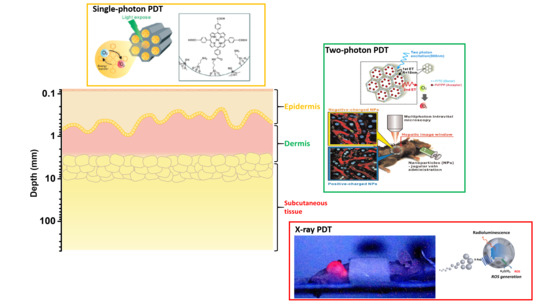
:1. Introduction
2. Single-Photon PDT
3. Two-Photon PDT
4. X-ray PDT
5. Encapsulation of PS in NP
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chabner, B.A.; Roberts, T.G., Jr. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Dart, A. Less is more. Nat. Rev. Cancer 2016, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.L.K.; Yeo, R.; Yeoh, K.W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Voon, S.H.; Kiew, L.V.; Lee, H.B.; Lim, S.H.; Noordin, M.I.; Kamkaew, A.; Burgess, K.; Chung, L.Y. In Vivo Studies of Nanostructure-Based Photosensitizers for Photodynamic Cancer Therapy. Small 2014, 10, 4993–5013. [Google Scholar] [CrossRef]
- Josefsen LB, B.R. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Lee, H.; Lee, S.; Min, K.H.; Kim, M.S.; Lee, D.S.; Choi, Y.; Kwon, I.C.; Kim, K.; Jeong, S.Y. In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem. Commun. 2010, 46, 5668–5670. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Wang, S.-B.; Lei, Q.; Zhu, J.-Y.; Zhang, X.-Z. Ratiometric Biosensor for Aggregation-Induced Emission-Guided Precise Photodynamic Therapy. ACS Nano 2015, 9, 10268–10277. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Wang, S.; Zhou, Z.; Li, Z.; Wang, Z.; Zhang, C.; Yue, X.; Niu, G.; Yang, M.; et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials 2013, 34, 4643–4654. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunakaran, S.C.; Babu, P.S.S.; Madhuri, B.; Marydasan, B.; Paul, A.K.; Nair, A.S.; Rao, K.S.; Srinivasan, A.; Chandrashekar, T.K.; Rao, C.M.; et al. In Vitro Demonstration of Apoptosis Mediated Photodynamic Activity and NIR Nucleus Imaging through a Novel Porphyrin. ACS Chem. Biol. 2013, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, X.; Zeng, Q.; Zhang, Y.; Tu, L.; Liu, T.; Kong, X.; Wang, Y.; Cao, F.; Lambrechts, S.A.G.; et al. Covalently Assembled NIR Nanoplatform for Simultaneous Fluorescence Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano 2012, 6, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kuang, J.; Li, C.X.; Zhang, M.; Zheng, D.; Zeng, X.; Liu, C.; Zhang, X.Z. Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication. ACS Nano 2018, 12, 1978–1989. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543. [Google Scholar] [CrossRef]
- Couleaud, P.; Morosini, V.; Frochot, C.; Richeter, S.; Raehm, L.; Durand, J.-O. Silica-based nanoparticles for photodynamic therapy applications. Nanoscale 2010, 2, 1083–1095. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.-L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Y.A.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Controll. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Kamkaew, A.; Burgess, K.; Kiew, L.V.; Chung, L.Y.; Lee, H.B. Small Molecules for Active Targeting in Cancer. Med. Res. Rev. 2016, 36, 494–575. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Controll. Release 2008, 132, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Controll. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Zhan, Q.; Qian, J.; Liang, H.; Somesfalean, G.; Wang, D.; He, S.; Zhang, Z.; Andersson-Engels, S. Using 915 nm Laser Excited Tm3+/Er3+/Ho3+-Doped NaYbF4 Upconversion Nanoparticles for in Vitro and Deeper in Vivo Bioimaging without Overheating Irradiation. ACS Nano 2011, 5, 3744–3757. [Google Scholar] [CrossRef]
- Simpson, C.R.; Kohl, M.; Essenpreis, M.; Cope, M. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys. Med. Biol. 1998, 43, 2465–2478. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef]
- Scaffidi, J.P.; Gregas, M.K.; Lauly, B.; Zhang, Y.; Vo-Dinh, T. Activity of Psoralen-Functionalized Nanoscintillators against Cancer Cells upon X-ray Excitation. ACS Nano 2011, 5, 4679–4687. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370. [Google Scholar] [CrossRef]
- Mallidi, S.A.S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lee, C.-H.; Yang, C.-S.; Tseng, F.-G.; Mou, C.-Y.; Lo, L.-W. Mesoporous silica nanoparticles functionalized with an oxygen-sensing probe for cell photodynamic therapy: Potential cancer theranostics. J. Mater. Chem. 2009, 19, 1252–1257. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lee, C.-H.; Chen, M.-C.; Souris, J.S.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Chen, C.-T.; Lo, L.-W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics-the trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157. [Google Scholar] [CrossRef]
- Park, D.J.; Min, K.H.; Lee, H.J.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Lee, S.C. Photosensitizer-loaded bubble-generating mineralized nanoparticles for ultrasound imaging and photodynamic therapy. J. Mater. Chem. B 2016, 4, 1219–1227. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McPherson, S.; DeGraff, W.; Gamson, J.; Zabell, A.; Russo, A. Oxygen Dependence of Hematoporphyrin Derivative-induced Photoinactivation of Chinese Hamster Cells. Cancer Res. 1985, 45, 2008–2011. [Google Scholar] [PubMed]
- Giaccia, A.J.; Simon, M.C.; Johnson, R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004, 18, 2183–2194. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721. [Google Scholar] [CrossRef]
- Henderson, B.W.; Fingar, V.H. Relationship of Tumor Hypoxia and Response to Photodynamic Treatment in an Experimental Mouse Tumor. Cancer Res. 1987, 47, 3110–3114. [Google Scholar]
- Brown, J.M. Tumor hypoxia in cancer therapy. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 2007; Volume 435, pp. 295–321. [Google Scholar]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-Activatable and O2-Evolving Nanoparticles for Highly Efficient and Selective Photodynamic Therapy against Hypoxic Tumor Cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S.H.; Hyeon, T. Continuous O2-Evolving MnFe2O4 Nanoparticle-Anchored Mesoporous Silica Nanoparticles for Efficient Photodynamic Therapy in Hypoxic Cancer. J. Am. Chem. Soc. 2017, 139, 10992–10995. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317. [Google Scholar] [CrossRef]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morère, A.; et al. Mannose-Functionalized Mesoporous Silica Nanoparticles for Efficient Two-Photon Photodynamic Therapy of Solid Tumors. Angew. Chem. Int. Ed. 2011, 50, 11425–11429. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Hsieh, C.-C.; Chen, N.-T.; Chu, C.-H.; Huang, C.-M.; Chou, P.-T.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Well-defined mesoporous nanostructure modulates three-dimensional interface energy transfer for two-photon activated photodynamic therapy. Nano. Today 2011, 6, 552–563. [Google Scholar] [CrossRef]
- Velusamy, M.; Shen, J.-Y.; Lin, J.T.; Lin, Y.-C.; Hsieh, C.-C.; Lai, C.-H.; Lai, C.-W.; Ho, M.-L.; Chen, Y.-C.; Chou, P.-T.; et al. A New Series of Quadrupolar Type Two-Photon Absorption Chromophores Bearing 11, 12-Dibutoxydibenzo[a,c]-phenazine Bridged Amines; Their Applications in Two-Photon Fluorescence Imaging and Two-Photon Photodynamic Therapy. Adv. Funct. Mater. 2009, 19, 2388–2397. [Google Scholar] [CrossRef]
- Kim, S.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Pandey, R.K.; Prasad, P.N. Organically Modified Silica Nanoparticles Co-encapsulating Photosensitizing Drug and Aggregation-Enhanced Two-Photon Absorbing Fluorescent Dye Aggregates for Two-Photon Photodynamic Therapy. J. Am. Chem. Soc. 2007, 129, 2669–2675. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional Gold Nanorods: Synthesis, Self-Assembly, and Sensing Applications. Adv. Mater. 2012, 24, 4811–4841. [Google Scholar] [CrossRef]
- Molinaro, C.; El Harfouch, Y.; Palleau, E.; Eloi, F.; Marguet, S.; Douillard, L.; Charra, F.; Fiorini-Debuisschert, C. Two-Photon Luminescence of Single Colloidal Gold Nanorods: Revealing the Origin of Plasmon Relaxation in Small Nanocrystals. J. Phy. Chem. C 2016, 120, 23136–23143. [Google Scholar] [CrossRef] [Green Version]
- Gaser, N.A.; Raffaella, M.; Silvia, D.; Marta, D.A.; Marco Scotto, D.A.; Teresa, P.; Alberto, D. PEGylated gold nanorods as optical trackers for biomedical applications: An in vivo and in vitro comparative study. Nanotechnology 2016, 27, 255101. [Google Scholar]
- Chen, N.T.; Tang, K.C.; Chung, M.F.; Cheng, S.H.; Huang, C.M.; Chu, C.H.; Chou, P.T.; Souris, J.S.; Chen, C.T.; Mou, C.Y.; et al. Enhanced Plasmonic Resonance Energy Transfer in Mesoporous Silica-Encased Gold Nanorod for Two-Photon-Activated Photodynamic Therapy. Thernostics 2014, 4, 798–807. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Shen, X.; Li, L.; Guan, Z.; Gao, N.; Yuan, P.; Yao, S.Q.; Xu, Q.-H.; Xu, G.Q. Gold nanorods as dual photo-sensitizing and imaging agents for two-photon photodynamic therapy. Nanoscale 2012, 4, 7712–7719. [Google Scholar] [CrossRef]
- Wang, C.; Volotskova, O.; Lu, K.; Ahmad, M.; Sun, C.; Xing, L.; Lin, W. Synergistic Assembly of Heavy Metal Clusters and Luminescent Organic Bridging Ligands in Metal–Organic Frameworks for Highly Efficient X-ray Scintillation. J. Am. Chem. Soc. 2014, 136, 6171–6174. [Google Scholar] [CrossRef]
- Kirakci, K.; Kubat, P.; Fejfarova, K.; Martincik, J.; Nikl, M.; Lang, K. X-ray Inducible Luminescence and Singlet Oxygen Sensitization by an Octahedral Molybdenum Cluster Compound: A New Class of Nanoscintillators. Inorg. Chem. 2016, 55, 803–809. [Google Scholar] [CrossRef]
- Osakada, Y.; Pratx, G.; Sun, C.; Sakamoto, M.; Ahmad, M.; Volotskova, O.; Ong, Q.; Teranishi, T.; Harada, Y.; Xing, L.; et al. Hard X-ray-induced optical luminescence via biomolecule-directed metal clusters. Chem. Commun. 2014, 50, 3549–3551. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zou, X.; Chen, W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1501–1508. [Google Scholar] [CrossRef]
- Generalov, R.; Kuan, W.B.; Chen, W.; Kristensen, S.; Juzenas, P. Radiosensitizing effect of zinc oxide and silica nanocomposites on cancer cells. Colloids Surf. B. 2015, 129, 79–86. [Google Scholar] [CrossRef]
- Clement, S.; Chen, W.; Anwer, A.G.; Goldys, E.M. Verteprofin conjugated to gold nanoparticles for fluorescent cellular bioimaging and X-ray mediated photodynamic therapy. Microchim. Acta 2017, 184, 1765–1771. [Google Scholar] [CrossRef]
- Elmenoufy, A.H.; Tang, Y.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Yang, W.; Read, P.W.; Mi, J.; Baisden, J.M.; Reardon, K.A.; Larner, J.M.; Helmke, B.P.; Sheng, K. Semiconductor nanoparticles as energy mediators for photosensitizer-enhanced radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 633–635. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Wang, G.; Qin, Z.; Wang, X.; Zhao, G.; Ma, Q.; Zhu, L. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials 2017, 112, 324. [Google Scholar] [CrossRef]
- Lucky, S.S.; Idris, N.M.; Huang, K.; Kim, J.; Li, Z.; Thong, P.S.; Xu, R.; Soo, K.C.; Zhang, Y. In vivo biocompatibility, biodistribution and therapeutic efficiency of titania coated upconversion nanoparticles for photodynamic therapy of solid oral cancers. Theranostics 2016, 6, 1844. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145. [Google Scholar] [CrossRef]
- Lucky, S.S.; Muhammad Idris, N.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano 2015, 9, 191. [Google Scholar] [CrossRef]
- Huang, Y.; Qiu, F.; Shen, L.; Chen, D.; Su, Y.; Yang, C.; Li, B.; Yan, D.; Zhu, X. Combining two-photon-activated fluorescence resonance energy transfer and near-infrared photothermal effect of unimolecular micelles for enhanced photodynamic therapy. ACS Nano 2016, 10, 10489. [Google Scholar] [CrossRef]
- Guo, L.; Ge, J.; Liu, Q.; Jia, Q.; Zhang, H.; Liu, W.; Niu, G.; Liu, S.; Gong, J.; Hackbarth, S.; et al. Versatile Polymer nanoparticles as two-photon-triggered photosensitizers for simultaneous cellular, deep-tissue imaging, and photodynamic therapy. Adv. Healthc. Mater. 2017, 6, 1601431. [Google Scholar] [CrossRef]
- Yang, G.G.; Hao, L.; Cao, Q.; Zhang, H.; Yang, J.; Ji, L.N.; Mao, Z.W. Three-in-one self-assembled nanocarrier for dual-drug delivery, two-photon imaging, and chemo-photodynamic synergistic therapy. ACS Appl. Mater. Interfaces 2018, 10, 28301. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.D.; Chuang, Y.J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano. Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [Green Version]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: Determination of singlet oxygen quantum yield. Sci. Rep. 2016, 6, 19954. [Google Scholar] [CrossRef]
- Tang, Y.A.; Hu, J.; Elmenoufy, A.H.; Yang, X. Highly Efficient FRET System Capable of Deep Photodynamic Therapy Established on X-ray Excited Mesoporous LaF3:Tb Scintillating Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 12261–12269. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Wang, S.; Joly, A.G. Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation. Appl. Phys. Lett. 2008, 92, 043901. [Google Scholar] [CrossRef]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef]
- Kaščáková, S.; Giuliani, A.; Lacerda, S.; Pallier, A.; Mercère, P.; Tóth, É.; Réfrégiers, M. X-ray-induced radiophotodynamic therapy (RPDT) using lanthanide micelles: Beyond depth limitations. Nano. Res. 2015, 8, 2373–2379. [Google Scholar] [CrossRef]
- Homayoni, H.; Jiang, K.; Zou, X.; Hossu, M.; Rashidi, L.H.; Chen, W. Enhancement of protoporphyrin IX performance in aqueous solutions for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2015, 12, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhao, K.; Bu, W.; Ni, D.; Liu, Y.; Feng, J.; Shi, J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 2015, 54, 1770–1774. [Google Scholar] [CrossRef]
- Ma, L.; Zou, X.; Bui, B.; Chen, W.; Song, K.H.; Solberg, T. X-ray excited ZnS:Cu,Co afterglow nanoparticles for photodynamic activation. Appl. Phys. Lett. 2014, 105, 013702. [Google Scholar] [CrossRef]
- Abliz, E.; Collins, J.E.; Bell, H.; Tata, D.B. Novel applications of diagnostic X-rays in activating a clinical photodynamic drug: Photofrin II through X-ray induced visible luminescence from “rare-earth” formulated particles. J. X-ray Sci. Technol. 2011, 19, 521–530. [Google Scholar]
- Rossi, F.; Bedogni, E.; Bigi, F.; Rimoldi, T.; Cristofolini, L.; Pinelli, S.; Alinovi, R.; Negri, M.; Dhanabalan, S.C.; Attolini, G.; et al. Porphyrin conjugated SiC/SiOx nanowires for X-ray-excited photodynamic therapy. Sci. Rep. 2015, 5, 7606. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Busch, T.M.; Wileyto, E.P.; Emanuele, M.J.; Del Piero, F.; Marconato, L.; Glatstein, E.; Koch, C.J. Photodynamic therapy creates fluence rate-dependent gradients in the intratumoral spatial distribution of oxygen. Cancer Res. 2002, 62, 7273–7279. [Google Scholar]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Moen, I.; Stuhr, L.E. Hyperbaric oxygen therapy and cancer--a review. Target. Oncol. 2012, 7, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wei, Y.; Xing, D.; Chen, Q. Increasing the efficiency of photodynamic therapy by improved light delivery and oxygen supply using an anticoagulant in a solid tumor model. Lasers Surg. Med. 2010, 42, 671–679. [Google Scholar] [CrossRef]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Wang, F.; Zamora, G.; Trinidad, A.; Marcu, L.; Cherry, S.; Hirschberg, H. Ultra Low Fluence Rate Photodynamic Therapy: Simulation of Light Emitted by the Cerenkov Effect. In Proceedings of the Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics, San Francisco, CA, USA, 1–6 February 2014; SPIE: Bellingham, WA, USA, 2014. [Google Scholar]
- Duan, L.; Yan, X.; Wang, A.; Jia, Y.; Li, J. Highly Loaded Hemoglobin Spheres as Promising Artificial Oxygen Carriers. ACS Nano 2012, 6, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-P.; Wu, T.-H.; Lin, Y.-L.; Liu, C.-Y.; Wang, S.; Lin, S.-Y. Tailoring Enzyme-Like Activities of Gold Nanoclusters by Polymeric Tertiary Amines for Protecting Neurons Against Oxidative Stress. Small 2016, 12, 4127–4135. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X.; Cheng, Z.; Lian, H.; Li, C.; Lin, J. UV-Emitting Upconversion-Based TiO2 Photosensitizing Nanoplatform: Near-Infrared Light Mediated in Vivo Photodynamic Therapy via Mitochondria-Involved Apoptosis Pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Shaffer, T.M.; Pratt, E.C.; Grimm, J. Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol. 2017, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Frackowiak, R.; Brzózka, Z.; Wilk, K.A. The effect of anionic dicephalic surfactants on fabrication of varied-core nanocarriers for sustained release of porphyrin photosensitizers. J. Photochem. Photobiol. B. 2017, 166, 169. [Google Scholar] [CrossRef]
- Bazylińska, U.; Wawrzyńczyk, D. Encapsulation of TOPO stabilized NaYF4:Er3+,Yb3+ nanoparticles in biocompatible nanocarriers: Synthesis, optical properties and colloidal stability. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 556. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Schmidt, J.; Talmon, Y.; Murgia, S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J. Colloid. Interface Sci. 2018, 15, 163. [Google Scholar] [CrossRef] [PubMed]




| Vehicle | PS Encapsulated | Dose | Mechanism | Remarks | Ref. |
|---|---|---|---|---|---|
| Manganese ferrite MS NP | Ce6 | 8 mM 200 μL (i.v) | Single photon (<1 cm) | Dramatically inhibited tumor growth | [45] |
| Poly(d,l-lactic-co-glycolic acid) (PLGA) | MB | 10 mg/kg (i.v) | Single photon (<1 cm) | Complete response in NP with PDT group | [44] |
| Perfluorocarbon | IR780 | 7.8 μg IR780 (i.t) | Single photon (<1 cm) | Inhibited 80% of tumor growth | [68] |
| Manganese dioxide NP | Indocyanine green | 3.6 mg/mL (i.v) | Single photon (<1 cm) | Complete response in NP with PDT group | [69] |
| NaYF4:Yb,Tm | TiO2 | 0.1 g/tumor (i.t) | Single photon a (1–2 cm) | 50% of the animals surviving up to 45 and 55 days | [70] |
| NaYF4:Yb3+, Er3+ | graphene quantum dot | Single photon a (1–2 cm) | Tumor inhibition efficacy ~70.2% | [71] | |
| NaYF4 | Ce6 | 32 mg/kg (i.t) | Single photon a (1–2 cm) | Tumors on 70% mice disappeared in two weeks | [72] |
| NaYF4:Yb,Tm @SiO2 | TiO2 | 0.1 g/tumor (i.t) | Single photon a (1–2 cm) | Inhibited 87.5% of tumor growth | [73] |
| MS NP | PS22 | 16 mg/kg (i.v.) | Two photon (≥2 cm) b | Inhibited 71% of tumor growth | [49] |
| MS-Encased Au NR | PdTPP | 16 mg/kg (i.t) | Two photon (≥2 cm) b | Inhibited 77% of tumor growth | [57] |
| Hyperbranched polymer HCP@HPE | Ce6 | 0.10 mmol/kg Chlorin e6 (i.v) | Two photon (≥2 cm) b | 87 % of tumor growth is suppressed compared to control | [74] |
| DSPE-PEG 2000 | PT2 | 100 μL, 500 μg/mL (i.v) | Two photon (≥2 cm) b | No apparent tumor growth was observed for 18 days | [75] |
| RuCD | 5-Fu | 25 mg/kg (i.t) | Two photon (≥2 cm) b | Tumor volume decreased by 85% compared to control | [76] |
| Nanosystem | Size | PS | Attachment Strategy | X-ray Doses | Exp. Subject | Ref. |
|---|---|---|---|---|---|---|
| MC540-SAO:Eu@mSiO2 | 400 nm | MC540 | Pore loading | 0.5 Gy, 50 kV | H1299 (in vitro, iv vivo) | [77,78] |
| U87MG xenograft | ||||||
| CeF3 | 7–11 nm | VP | Physical loading | 6 Gy, 8 keV, 30 keV, 6 MeV | Panc1 (in vitro) | [79] |
| (n-Bu4N)2[Mo6I8(OOCC10H15)6] | 50 nm | self | Encapsulated | 100 keV | N/A | [60] |
| LaF3:Tb | 25–44 nm | RB | Pore loading | 75 kV, 20 mA | N/A | [80] |
| 50–150 nm | RB | Covalent binding | 75 kV, 20 mA | N/A | [65] | |
| 15 nm | MTCP | Physical loading | 13.2 Gy, 250 keV | N/A | [81] | |
| LaF3:Ce | 2 μm | PPIX | Physical loading | 2 Gy, 90 kV, 5 mA | PC-3 (in vitro) | [82] |
| ZnO/SiO2 | 80–100 nm | ZnO | Coating | 2-10 Gy, 200 kVp, 20 mA | LNCaP and Du145 (in vitro) | [63] |
| GdEuC12 micelle | 4.6 nm | Hyp | Physical loading | 400 mA | Hela (in vitro) | [83] |
| N/A | PPIX | Covalent binding | 8 Gy | PC-3 (in vitro) | [84] | |
| LiYF4:Ce@SiO2 | 50 nm | ZnO | Coating | 8 Gy, 220 keV | HeLa xenograft | [85] |
| TiO2-Tf-Tc | 108 nm | TiO2 | N/A | Cerenkov radiation | HT1080 xenograft | [33] |
| Cu-Cy | 50–100 nm | self | 5 Gy | MCF-7 xenograft | [62] | |
| ZnS:Cu,Co | 4 nm | TBrRh123 | Covalent binding | 2 Gy, 120 kVp | PC-3 (in vitro) | [86] |
| Tb2O3 | 10 nm | porphorin | Covalent binding | 44 kV, 40 mA, 5.4 mGy/s | N/A | [66] |
| Y2O3 | 12 nm | PS | Covalent binding | 2 Gy, 160 or 320 kVp | PC-3 (in vitro) | [32] |
| Gd2O2S:Tb | 20 μm | Photo II | Colocation | 130 kVp, 20 mA | Human glioblastoma | [87] |
| SiC/SiOx nanowires | 20 nm | H2TCPP | Covalent binding | 2 Gy, 6 MV | A549 (in vitro) | [88] |
| AuNPs | 12 nm | verteporfin | Covalent binding | 6 Gy, 6 MV | Panc 1 (in vitro) | [64] |
| CdSe@ZnS | 2.1 nm | N/A | N/A | 100–600 cGy/min, 6 MV | H460 (in vitro) | [67] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasubramanian, M.; Chuang, Y.C.; Lo, L.-W. Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers. Molecules 2019, 24, 520. https://doi.org/10.3390/molecules24030520
Sivasubramanian M, Chuang YC, Lo L-W. Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers. Molecules. 2019; 24(3):520. https://doi.org/10.3390/molecules24030520
Chicago/Turabian StyleSivasubramanian, Maharajan, Yao Chen Chuang, and Leu-Wei Lo. 2019. "Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers" Molecules 24, no. 3: 520. https://doi.org/10.3390/molecules24030520
APA StyleSivasubramanian, M., Chuang, Y. C., & Lo, L. -W. (2019). Evolution of Nanoparticle-Mediated Photodynamic Therapy: From Superficial to Deep-Seated Cancers. Molecules, 24(3), 520. https://doi.org/10.3390/molecules24030520