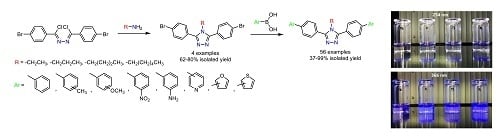
3.2.3. General Procedure for Conventional Suzuki Cross-Coupling Reactions. Synthesis of 4-Alkyl-3,5-bis(4-arylphenyl)-4H-1,2,4-triazoles 5a–n, 6a–n, 7a–n, 8a–n
To a mixture of 4-alkyl-3,5-bis(4-bromophenyl)-4H-1,2,4-triazole (3a–d, 1.00 mmol), the corresponding boronic acid (4a–n, 2.50 mmol), palladium catalyst Pd(PPh3)4 (0.058 g, 0.05 mmol), phase transfer catalyst NBu4Br (0.032 g, 0.10 mmol) and base K2CO3 (1.382 g, 10.00 mmol), toluene (9 mL), H2O (6 mL) and EtOH (3 mL) were added. The mixture was heated under reflux in an oil bath (130 °C) for 4–12 h (reaction was monitored by TLC). After cooling, chloroform (50 mL) was added and the solution transferred to a separating funnel. The aqueous layer was extracted with chloroform (2 × 10 mL). The combined organic layers were filtered through a silica gel plug (10 mL), which was then flushed with CHCl3/EtOAc (5:1 v/v). The filtrate was dried over MgSO4 and then concentrated using a rotary evaporator. The product was precipitated using EtOAc (5 mL), filtered, washed with fresh EtOAc and air-dried to give the corresponding pure 4-alkyl-3,5-bis(4-arylphenyl)-4H-1,2,4-triazole (5a–n, 6a–n, 7a–n, 8a–n).
3,5-Bis(biphenyl-4-yl)-4-ethyl-4H-1,2,4-triazole (5a). Beige solid in 87% yield, 0.348 g, m.p. 229–231 °C; UV (CH2Cl2) λmax 281.0 nm (ε⋅10−3 36.0 cm−1M−1); IR (ATR) ν: 3029, 2965, 1471, 1456, 1445, 1420, 1340, 1083, 1076, 1007, 968, 847, 765, 750, 730, 695, 598 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.16 (t, J = 7.2 Hz, 3H, CH3), 4.23 (q, J = 7.2 Hz, 2H, CH2), 7.39 (t, J = 7.2 Hz, 2H, ArH), 7.47 (t, J = 7.2 Hz, 4H, ArH), 7.64 (d, J = 7.2 Hz, 4H, ArH), 7.74–7.78 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 40.0, 127.2, 127.6, 127.9, 128.3, 128.9, 129.3, 140.0, 142.9, 155.1; HRMS m/z calcd for (C28H23N3 + H+): 402.1965; found: 402.1963.
4-Ethyl-3,5-bis(2′-methylbiphenyl-4-yl)-4H-1,2,4-triazole (5b). White solid in 85% yield, 0.366 g, m.p. 247–250 °C; UV (CH2Cl2) λmax 268.0 nm (ε⋅10−3 32.6 cm−1M−1); IR (ATR) ν: 3060, 3016, 1473, 1454, 1425, 1379, 1109, 1051, 1007, 968, 849, 842, 835, 807, 765, 743, 729 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.22 (t, J = 7.2 Hz, 3H, CH3), 2.32 (s, 6H, 2 × CH3), 4.27 (q, J = 7.2 Hz, 2H, CH2), 7.28–7.31 (m, 8H, ArH), 7.50 (d, J = 8.4 Hz, 4H, ArH), 7.75 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 16.0, 20.4, 40.0, 125.9, 126.2, 127.7, 128.7, 129.6, 129.8, 130.5, 135.3, 140.9, 143.8, 155.2; HRMS m/z calcd for (C30H27N3 + H+): 430.2278; found: 430.2274.
4-Ethyl-3,5-bis(3′-methylbiphenyl-4-yl)-4H-1,2,4-triazole (5c). White solid in 97% yield, 0.417 g, m.p. 194–195 °C; UV (CH2Cl2) λmax 283.0 nm (ε⋅10−3 41.3 cm−1M−1); IR (ATR) ν: 3015, 2983, 1740, 1474, 1418, 1371, 1337, 1236, 1113, 1083, 1042, 1018, 968, 853, 842, 780, 755, 730, 609 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.17 (t, J = 7.2 Hz, 3H, CH3), 2.45 (s, 6H, 2 × CH3), 4.23 (q, J = 7.2 Hz, 2H, CH2), 7.22 (d, J = 7.6 Hz, 2H, ArH), 7.37 (t, J = 7.6 Hz, 2H, ArH), 7.45–7.47 (m, 4H, ArH), 7.61–7.63 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 21.5, 40.0, 124.3, 126.5, 127.6, 127.9, 128.7, 128.8, 129.3, 138.6, 140.1, 143.0, 155.2; HRMS m/z calcd for (C30H27N3 + H+): 430.2278; found: 430.2279.
4-Ethyl-3,5-bis(2′,6′-dimethylbiphenyl-4-yl)-4H-1,2,4-triazole (5d). White solid in 90% yield, 0.412 g, m.p. 326–329 °C; UV (CH2Cl2) λmax 259.0 nm (ε⋅10−3 28.5 cm−1M−1); IR (ATR) ν: 2984, 2917, 1482, 1466, 1442, 1426, 1384, 1163, 1112, 1004, 963, 846, 773, 728, 719 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.22 (t, J = 7.2 Hz, 3H, CH3), 2.08 (s, 12H, 4 × CH3), 4.29 (q, J = 7.2 Hz, 2H, CH2), 7.14 (d, J = 7.2 Hz, 4H, ArH), 7.19–7.23 (m, 2H, ArH), 7.34 (d, J = 8.4 Hz, 4H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.8, 20.8, 40.0, 126.2, 127.4, 129.1, 129.8, 135.8, 140.8, 143.2, 155.3; HRMS m/z calcd for (C32H31N3 + H+): 458.2591; found: 458.2593.
4-Ethyl-3,5-bis(2′-methoxybiphenyl-4-yl)-4H-1,2,4-triazole (5e). Creamy solid in 91% yield, 0.420 g, m.p. 189–190 °C; UV (CH2Cl2) λmax 272.0 nm (ε⋅10−3 33.0 cm−1M−1) and 296.0 (33.2); IR (ATR) ν: 3016, 2941, 2838, 1595, 1493, 1470, 1432, 1400, 1258, 1244, 1123, 1053, 1021, 1004, 964, 855, 835, 803, 749, 734, 727 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.20 (t, J = 7.2 Hz, 3H, CH3), 3.85 (s, 6H, 2 × OCH3), 4.26 (q, J = 7.2 Hz, 2H, CH2), 7.02 (d, J = 8.4 Hz, 2H, ArH), 7.07 (t, J = 7.6 Hz, 2H, ArH), 7.35–7.40 (m, 4H, ArH), 7.71 (d, J = 8.4 Hz, 4H, ArH), 7.75 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 16.0, 40.0, 55.6, 111.4, 121.0, 126.2, 128.5, 129.2, 129.6, 130.5, 130.8, 140.3, 155.3, 156.5; HRMS m/z calcd for (C30H27N3O2 + H+): 462.2176; found: 462.2175.
4-Ethyl-3,5-bis(3′-methoxybiphenyl-4-yl)-4H-1,2,4-triazole (5f). White solid in 99% yield, 0.457 g, m.p. 191–192 °C; UV (CH2Cl2) λmax 282.0 nm (ε⋅10−3 31.6 cm−1M−1); IR (ATR) ν: 3007, 2953, 2836, 1737, 1609, 1583, 1474, 1437, 1417, 1294, 1268, 1212, 1170, 1114, 1095, 1082, 1053, 1030, 1014, 853, 840, 768, 753, 730 cm−1; 1H-NMR (400 MHz, CDCl3): δ1.18 (t, J = 7.2 Hz, 3H, CH3), 3.90 (s, 6H, 2 × OCH3), 4.24 (q, J = 7.2 Hz, 2H, CH2), 6.95 (dd, J = 8.0 and 2.4 Hz, 2H, ArH), 7.19 (t, J = 2.4 Hz, 2H, ArH), 7.23–7.26 (m, 2H, ArH), 7.41 (t, J = 8.0 Hz, 2H, ArH), 7.74–7.78 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 40.0, 55.4, 113.0, 113.3, 119.7, 126.8, 127.7, 129.3, 130.0, 141.6, 142.7, 155.1, 160.1; HRMS m/z calcd for (C30H27N3O2 + H+): 462.2176; found: 462.2170.
4-Ethyl-3,5-bis(3′-nitrobiphenyl-4-yl)-4H-1,2,4-triazole (5g). Yellow solid in 94% yield, 0.462 g, m.p. 246–248 °C; UV (CH2Cl2) λmax 275.0 nm (ε⋅10−3 57.4 cm−1M−1); IR (ATR) ν: 3076, 1517, 1485, 1469, 1346, 1287, 1103, 1086, 1010, 963, 877, 854, 837, 804, 776, 729, 798, 692 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.20 (t, J = 7.2 Hz, 3H, CH3), 4.26 (q, J = 7.2 Hz, 2H, CH2), 7.69 (t, J = 8.0 Hz, 2H, ArH), 7.83 (d, J = 8.4 Hz, 4H, ArH), 7.87 (d, J = 8.4 Hz, 4H, ArH), 8.00 (d, J = 8.0 Hz, 2H, ArH), 8.28 (d, J = 8.0 Hz, 2H, ArH), 8.54 (s, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 40.1, 122.0, 122.7, 127.8, 127.9, 129.7, 130.0, 133.0, 140.4, 141.7, 148.9, 154.9; HRMS m/z calcd for (C28H21N5O4 + H+): 492.1666; found: 492.1678.
3,5-Bis(3′-aminobiphenyl-4-yl)-4-ethyl-4H-1,2,4-triazole (5h). Beige solid in 99% yield, 0.428 g, m.p. 232–234 °C; UV (CH2Cl2) λmax 258.0 nm (ε⋅10−3 28.5 cm−1M−1) and 280.0 (32.2); IR (ATR) ν: 3455, 3426, 3348, 3305, 3188, 3046, 1621, 1598, 1566, 1473, 1448, 1426, 1313, 971, 888, 867, 840, 777, 748 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.16 (t, J = 7.2 Hz, 3H, CH3), 3.80 (br.s, 4H, 2 × NH2), 4.22 (q, J = 7.2 Hz, 2H, CH2), 6.72 (d, J = 7.6 Hz, 2H, ArH), 6.97 (s, 2H, ArH), 7.05 (d, J = 7.6 Hz, 2H, ArH), 7.27 (t, J = 7.6 Hz, 2H, ArH), 7.72-7.75 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 40.0, 113.8, 114.7, 117.6, 126.5, 127.5, 129.2, 129.9, 141.3, 143.0, 146.9, 155.2; HRMS m/z calcd for (C28H25N5 + H+): 432.2183; found: 432.2169.
4-Ethyl-3,5-bis[4-(pyridin-4-yl)phenyl]-4H-1,2,4-triazole (5i). Grey solid in 89% yield, 0.359 g, m.p. 248–251 °C; UV (CH2Cl2) λmax 280.0 nm (ε⋅10−3 31.0 cm−1M−1); IR (ATR) ν: 3067, 1594, 1541, 1474, 1411, 1221, 993, 969, 858, 808, 771, 751, 733, 664 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.19 (t, J = 7.2 Hz, 3H, CH3), 4.25 (q, J = 7.2 Hz, 2H, CH2), 7.57 (d, J = 6.0 Hz, 4H, ArH), 7.82 (d, J = 8.4 Hz, 4H, ArH), 7.85 (d, J = 8.4 Hz, 4H, ArH), 8.72 (d, J = 6.0 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 40.1, 121.6, 127.6, 128.3, 129.6, 139.9, 147.1, 150.5, 154.8; HRMS m/z calcd for (C26H21N5 + H+): 404.1870; found: 404.1868.
4-Ethyl-3,5-bis[4-(pyridin-3-yl)phenyl]-4H-1,2,4-triazole (5j). Yellow solid in 93% yield, 0.374 g, m.p. 199–202 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 54.8 cm−1M−1); IR (ATR) ν: 3025, 1464, 1433, 1416, 1383, 1025, 999, 971, 855, 848, 806, 772, 749, 712 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.19 (t, J = 7.2 Hz, 3H, CH3), 4.26 (q, J = 7.2 Hz, 2H, CH2), 7.42 (dd, J = 7.6 and 5.2 Hz, 2H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH), 7.84 (d, J = 8.4 Hz, 4H, ArH), 7.95 (d, J = 7.6 Hz, 2H, ArH), 8.66 (dd, J = 5.2 and 1.6 Hz, 2H, ArH), 8.93 (d, J = 1.6 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.9, 40.1, 123.7, 127.4, 127.7, 129.6, 134.4, 135.5, 139.6, 148.3, 149.1, 154.9; HRMS m/z calcd for (C26H21N5 + H+): 404.1870; found: 404.1870.
4-Ethyl-3,5-bis[4-(furan-2-yl)phenyl]-4H-1,2,4-triazole (5k). Creamy solid in 92% yield, 0.351 g, m.p. 236–237 °C; UV (CH2Cl2) λmax 310.0 nm (ε⋅10−3 53.0 cm−1M−1); IR (ATR) ν: 3114, 1615, 1493, 1471, 1189, 1158, 1083, 1007, 967, 905, 884, 848, 833, 809, 792, 744, 719 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.13 (t, J = 7.2 Hz, 3H, CH3), 4.19 (q, J = 7.2 Hz, 2H, CH2), 6.52 (dd, J = 3.6 and 2.0 Hz, 2H, ArH), 6.78 (d, J = 3.6 Hz, 2H, ArH), 7.53 (d, J = 2.0 Hz, 2H, ArH), 7.71 (d, J = 8.8 Hz, 4H, ArH), 7.83 (d, J = 8.8 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.7, 40.0, 106.4, 111.9, 124.1, 126.3, 129.2, 132.3, 142.8, 153.0, 155.1; HRMS m/z calcd for (C24H19N3O2 + H+): 382.1550; found: 382.1554.
4-Ethyl-3,5-bis[4-(furan-3-yl)phenyl]-4H-1,2,4-triazole (5l). Creamy solid in 91% yield, 0.347 g, m.p. 246–248 °C; UV (CH2Cl2) λmax 283.0 nm (ε⋅10−3 27.7 cm−1M−1); IR (ATR) ν: 3143, 2969, 1507, 1472, 1403, 1350, 1195, 1162, 1115, 1084, 1054, 1016, 923, 872, 843, 785, 760, 739 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.13 (t, J = 7.2 Hz, 3H, CH3), 4.18 (q, J = 7.2 Hz, 2H, CH2), 6.76 (dd, J = 1.6 and 0.8 Hz, 2H, ArH), 7.53 (t, J = 1.6 Hz, 2H, ArH), 7.65 (d, J = 8.8 Hz, 4H, ArH), 7.71 (d, J = 8.8 Hz, 4H, ArH), 7.83 (dd, J = 1.6 and 0.8 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.8, 39.9, 108.7, 125.7, 126.2, 126.3, 129.4, 134.2, 139.2, 144.1, 155.1; HRMS m/z calcd for (C24H19N3O2 + H+): 382.1550; found: 382.1548.
4-Ethyl-3,5-bis[4-(thiophen-2-yl)phenyl]-4H-1,2,4-triazole (5m). Creamy solid in 61% yield, 0.254 g, m.p. 239–242 °C; UV (CH2Cl2) λmax 311.0 nm (ε⋅10−3 43.2 cm−1M−1); IR (ATR) ν: 3089, 3004, 2961, 1470, 1428, 1353, 1260, 1216, 1194, 1116, 1081, 1012, 967, 959, 853, 843, 819, 785, 773, 740, 704, 693 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.14 (t, J = 7.2 Hz, 3H, CH3), 4.20 (q, J = 7.2 Hz, 2H, CH2), 7.12 (dd, J = 5.2 and 3.6 Hz, 2H, ArH), 7.35 (d, J = 5.2 Hz, 2H, ArH), 7.42 (d, J = 3.6 Hz, 2H, ArH), 7.70 (d, J = 8.4 Hz, 4H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.8, 40.0, 124.0, 125.8, 126.2, 126.5, 128.3, 129.4, 136.0, 143.1, 155.0; HRMS m/z calcd for (C24H19N3S2 + H+): 414.1093; found: 414.1077.
4-Ethyl-3,5-bis[4-(thiophen-3-yl)phenyl]-4H-1,2,4-triazole (5n). Beige solid in 90% yield, 0.372 g, m.p. 265–266 °C; UV (CH2Cl2) λmax 290.0 nm (ε⋅10−3 42.5 cm−1M−1); IR (ATR) ν: 3094, 2962, 1470, 1352, 1278, 1205, 1119, 1083, 1017, 968, 870, 843, 780, 760, 733, 695, 667, 631 cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.14 (t, J = 7.2 Hz, 3H, CH3), 4.20 (q, J = 7.2 Hz, 2H, CH2), 7.43-7.47 (m, 4H, ArH), 7.57 (dd, J = 2.8 and 1.6 Hz, 2H, ArH), 7.72 (d, J = 8.4 Hz, 4H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 15.8, 40.0, 121.3, 126.1, 126.3, 126.7, 126.8, 129.4, 137.4, 141.2, 155.1; HRMS m/z calcd for (C24H19N3S2 + H+): 414.1093; found: 414.1102.
3,5-Bis(biphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6a). Creamy solid in 94% yield, 0.391 g, m.p. 285–288 °C; UV (CH2Cl2) λmax 282.0 nm (ε⋅10−3 41.8 cm−1M−1); IR (ATR) ν: 3058, 2958, 2934, 2874, 1467, 1446, 1420, 1397, 1385, 1349, 1005, 969, 843, 802, 767, 745, 734, 724, 688 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.68 (t, J = 7.6 Hz, 3H, CH3), 1.50 (sext, J = 7.6 Hz, 2H, CH2), 4.16 (t, J = 7.6 Hz, 2H, CH2), 7.40 (t, J = 7.2 Hz, 2H, ArH), 7.49 (t, J = 7.2 Hz, 4H, ArH), 7.67 (d, J = 7.2 Hz, 4H, ArH), 7.75–7.79 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.6, 126.7, 127.1, 127.6, 127.9, 128.9, 129.3, 140.0, 142.8, 155.4; HRMS m/z calcd for (C29H25N3 + H+): 416.2121; found: 416.2120.
3,5-Bis(2′-methylbiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6b). White solid in 81% yield, 0.360 g, m.p. 230–232 °C; UV (CH2Cl2) λmax 268.0 nm (ε⋅10−3 37.9 cm−1M−1); IR (ATR) ν: 3052, 2961, 2873, 1474, 1425, 1382, 1112, 1093, 1007, 972, 864, 847, 768, 747, 722 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.71 (t, J = 7.2 Hz, 3H, CH3), 1.54 (sext, J = 7.2 Hz, 2H, CH2), 2.32 (s, 6H, 2 × CH3), 4.19 (t, J = 7.2 Hz, 2H, CH2), 7.28–7.31 (m, 8H, ArH), 7.50 (d, J = 8.0 Hz, 4H, ArH), 7.75 (d, J = 8.0 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 20.4, 23.5, 46.6, 125.9, 126.3, 127.7, 128.7, 129.6, 129.8, 130.5, 135.3, 140.9, 143.8, 155.5; HRMS m/z calcd for (C31H29N3 + H+): 444.2434; found: 444.2432.
3,5-Bis(3′-methylbiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6c). White solid in 91% yield, 0.404 g, m.p. 223–225 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 45.2 cm−1M−1); IR (ATR) ν: 3050, 2967, 2877, 1607, 1470, 1414, 1382, 1336, 1093, 1020, 969, 863, 852, 838, 775, 752, 725 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.68 (t, J = 7.6 Hz, 3H, CH3), 1.50 (sext, J = 7.6 Hz, 2H, CH2), 2.45 (s, 6H, 2 × CH3), 4.15 (t, J = 7.6 Hz, 2H, CH2), 7.22 (d, J = 7.6 Hz, 2H, ArH), 7.38 (t, J = 7.6 Hz, 2H, ArH), 7.45–7.48 (m, 4H, ArH), 7.74–7.77 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 21.5, 23.5, 46.6, 124.3, 126.6, 127.6, 127.9, 128.7, 128.9, 129.3, 138.6, 140.0, 142.9, 155.5; HRMS m/z calcd for (C31H29N3 + H+): 444.2434; found: 444.2430.
3,5-Bis(2′,6′-dimethylbiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6d). White solid in 94% yield, 0.444 g, m.p. 285–286 °C; UV (CH2Cl2) λmax 259.0 nm (ε⋅10−3 29.5 cm−1M−1); IR (ATR) ν: 3035, 2969, 1466, 1424, 1379, 1092, 1004, 973, 863, 841, 765, 751, 729 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.69 (t, J = 7.2 Hz, 3H, CH3), 1.53 (sext, J = 7.2 Hz, 2H, CH2), 2.07 (s, 12H, 4 × CH3), 4.20 (t, J = 7.2 Hz, 2H, CH2), 7.14 (d, J = 7.2 Hz, 4H, ArH), 7.19–7.23 (m, 2H, ArH), 7.33 (d, J = 8.4 Hz, 4H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 20.8, 23.4, 46.6, 99.1, 126.3, 127.4, 129.1, 129.8, 135.8, 140.8, 143.1, 155.6; HRMS m/z calcd for (C33H33N3 + H+): 472.2747; found: 472.2744.
3,5-Bis(2′-methoxybiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6e). White solid in 91% yield, 0.433 g, m.p. 198–199 °C; UV (CH2Cl2) λmax 273.0 nm (ε⋅10−3 27.7 cm−1M−1) and 296.0 (28.6); IR (ATR) ν: 3037, 2933, 2830, 1598, 1495, 1465, 1421, 1257, 1239, 1122, 1112, 1055, 1024, 1005, 860, 839, 803, 735, 721 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.71 (t, J = 7.6 Hz, 3H, CH3), 1.55 (sext, J = 7.6 Hz, 2H, CH2), 3.85 (s, 6H, 2 × OCH3), 4.18 (t, J = 7.6 Hz, 2H, CH2), 7.02 (d, J = 8.4 Hz, 2H, ArH), 7.07 (t, J = 7.6 Hz, 2H, ArH), 7.35-7.40 (m, 4H, ArH), 7.70 (d, J = 8.4 Hz, 4H, ArH), 7.74 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.6, 55.6, 111.4, 121.0, 126.3, 128.5, 129.2, 129.6, 130.0, 130.8, 140.3, 155.6, 156.5; HRMS m/z calcd for (C31H29N3O2 + H+): 476.2333; found: 476.2332.
3,5-Bis(3′-methoxybiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6f). White solid in 95% yield, 0.452 g, m.p. 193–195 °C; UV (CH2Cl2) λmax 283.0 nm (ε⋅10−3 35.5 cm−1M−1); IR (ATR) ν: 2969, 2836, 1600, 1592, 1560, 1463, 1418, 1301, 1219, 1166, 1050, 1029, 1016, 858, 869, 849, 840, 795, 778, 753, 744 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.68 (t, J = 7.6 Hz, 3H, CH3), 1.50 (sext, J = 7.6 Hz, 2H, CH2), 3.90 (s, 6H, 2 × OCH3), 4.15 (t, J = 7.6 Hz, 2H, CH2), 6.95 (dd, J = 8.0 and 2.4 Hz, 2H, ArH), 7.19 (t, J = 2.4 Hz, 2H, ArH), 7.23–7.26 (m, 2H, ArH), 7.40 (t, J = 8.0 Hz, 2H, ArH), 7.74–7.78 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.6, 55.4, 112.9, 113.3, 119.6, 126.9, 127.6, 129.3, 130.0, 141.5, 142.6, 155.4, 160.1; HRMS m/z calcd for (C31H29N3O2 + H+): 476.2333; found: 476.2334.
3,5-Bis(3′-nitrobiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6g). Creamy solid in 91% yield, 0.460 g, m.p. 291–293 °C; UV (CH2Cl2) λmax 276.0 nm (ε⋅10−3 47.3 cm−1M−1); IR (ATR) ν: 3034, 2969, 2872, 1521, 1488, 1468, 1344, 1294, 1104, 876, 852, 840, 802, 777, 759, 742, 723 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.70 (t, J = 7.2 Hz, 3H, CH3), 1.52 (sext, J = 7.2 Hz, 2H, CH2), 4.19 (t, J = 7.2 Hz, 2H, CH2), 7.68 (t, J = 7.6 Hz, 2H, ArH), 7.82 (d, J = 8.4 Hz, 4H, ArH), 7.86 (d, J = 8.4 Hz, 4H, ArH), 8.00 (dd, J = 7.6 and 2.0 Hz, 2H, ArH), 8.27 (dd, J = 7.6 and 2.0 Hz, 2H, ArH), 8.54 (t, J = 2.0 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.6, 46.8, 122.0, 122.7, 127.8, 128.0, 129.7, 130.0, 133.0, 137.2, 140.3, 141.7, 155.2; HRMS m/z calcd for (C29H23N5O4 + H+): 506.1823; found: 506.1811.
3,5-Bis(3′-aminobiphenyl-4-yl)-4-propyl-4H-1,2,4-triazole (6h). Beige solid in 99% yield, 0.428 g, m.p. 216–218 °C; UV (CH2Cl2) λmax 235.0 nm (ε⋅10−3 27.0 cm−1M−1), 258.0 (29.2) and 282.0 (33.9); IR (ATR) ν: 3441, 3407, 3336, 3143, 2961, 1601, 1586, 1564, 1479, 1431, 1324, 1233, 993, 893, 842, 775, 743, 714 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.67 (t, J = 7.2 Hz, 3H, CH3), 1.49 (sext, J = 7.2 Hz, 2H, CH2), 3.80 (br.s, 4H, 2x NH2), 4.13 (t, J = 7.2 Hz, 2H, CH2), 6.72 (dd, J = 8.0 and 2.0 Hz, 2H, ArH), 6.98 (t, J = 2.0 Hz, 2H, ArH), 7.05 (dd, J = 8.0 and 2.0 Hz, 2H, ArH), 7.27 (t, J = 8.0 Hz, 2H, ArH), 7.72–7.74 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.6, 113.8, 114.7, 117.5, 126.6, 127.5, 129.2, 129.9, 141.2, 143.0, 146.9, 155.5; HRMS m/z calcd for (C29H27N5 + H+): 446.2339; found: 446.2348.
4-Propyl-3,5-bis[4-(pyridin-4-yl)phenyl]-4H-1,2,4-triazole (6i). Creamy solid in 90% yield, 0.376 g, m.p. 237–240 °C; UV (CH2Cl2) λmax 281.0 nm (ε⋅10−3 36.5 cm−1M−1); IR (ATR) ν: 2957, 1596, 1541, 1471, 1408, 1347, 1217, 992, 969, 859, 812, 772, 752, 737 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.69 (t, J = 7.6 Hz, 3H, CH3), 1.50 (sext, J = 7.6 Hz, 2H, CH2), 4.17 (t, J = 7.6 Hz, 2H, CH2), 7.58 (d, J = 6.0 Hz, 4H, ArH), 7.81–7.84 (m, 8H, ArH), 8.73 (d, J = 6.0 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.7, 121.6, 127.6, 128.4, 129.6, 139.8, 147.1, 150.5, 155.2; HRMS m/z calcd for (C27H23N5 + H+): 418.2026; found: 418.2022.
4-Propyl-3,5-bis[4-(pyridin-3-yl)phenyl]-4H-1,2,4-triazole (6j). Creamy solid in 69% yield, 0.288 g, m.p. 212–215 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 40.6 cm−1M−1); IR (ATR) ν: 3035, 2965, 2875, 1470, 1426, 1414, 1383, 1339, 1025, 1001, 970, 851, 799, 749, 707 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.69 (t, J = 7.6 Hz, 3H, CH3), 1.51 (sext, J = 7.6 Hz, 2H, CH2), 4.18 (t, J = 7.6 Hz, 2H, CH2), 7.42 (dd, J = 8.0 and 4.8 Hz, 2H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH), 7.83 (d, J = 8.4 Hz, 4H, ArH), 7.95 (dt, J = 8.0 and 1.6 Hz, 2H, ArH), 8.66 (dd, J = 4.8 and 1.6 Hz, 2H, ArH), 8.93 (d, J = 1.6 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.7, 123.7, 127.5, 127.7, 129.6, 134.4, 135.5, 139.5, 148.3, 149.1, 155.2; HRMS m/z calcd for (C27H23N5 + H+): 418.2026; found: 418.2028.
3,5-Bis[4-(furan-2-yl)phenyl]-4-propyl-4H-1,2,4-triazole (6k). Beige solid in 96% yield, 0.379 g, m.p. 279–281 °C; UV (CH2Cl2) λmax 311.0 nm (ε⋅10−3 63.6 cm−1M−1); IR (ATR) ν: 2960, 1615, 1491, 1465, 1351, 1282, 1220, 1116, 1076, 1020, 1010, 903, 862, 839, 815, 805, 757, 735, 663 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.64 (t, J = 7.6 Hz, 3H, CH3), 1.45 (sext, J = 7.6 Hz, 2H, CH2), 4.10 (t, J = 7.6 Hz, 2H, CH2), 6.53 (dd, J = 3.6 and 2.0 Hz, 2H, ArH), 6.78 (d, J = 3.6 Hz, 2H, ArH), 7.53 (d, J = 2.0 Hz, 2H, ArH), 7.71 (d, J = 8.8 Hz, 4H, ArH), 7.83 (d, J = 8.8 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.4, 46.7, 106.4, 111.9, 124.1, 126.4, 129.2, 132.3, 142.8, 153.0, 155.4; HRMS m/z calcd for (C25H21N3O2 + H+): 396.1707; found: 396.1709.
3,5-Bis[4-(furan-3-yl)phenyl]-4-propyl-4H-1,2,4-triazole (6l). Creamy solid in 92% yield, 0.363 g, m.p. 299–303 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 35.3 cm−1M−1); IR (ATR) ν: 3141, 1507, 1471, 1351, 1162, 1115, 1055, 1015, 923, 872, 843, 785, 754, 739 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.65 (t, J = 7.6 Hz, 3H, CH3), 1.46 (sext, J = 7.6 Hz, 2H, CH2), 4.10 (t, J = 7.6 Hz, 2H, CH2), 6.77 (dd, J = 2.0 and 1.2 Hz, 2H, ArH), 7.53 (d, J = 2.0 Hz, 2H, ArH), 7.65 (d, J = 8.8 Hz, 4H, ArH), 7.70 (d, J = 8.8 Hz, 4H, ArH), 7.84 (d, J = 1.2 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.4, 46.6, 108.6, 125.7, 126.2, 126.3, 129.4, 134.2, 139.2, 144.1, 155.4; HRMS m/z calcd for (C25H21N3O2 + H+): 396.1707; found: 396.1710.
4-Propyl-3,5-bis[4-(thiophen-2-yl)phenyl]-4H-1,2,4-triazole (6m). Grey solid in 52% yield, 0.223 g, m.p. 283–285 °C; UV (CH2Cl2) λmax 312.0 nm (ε⋅10−3 36.4 cm−1M−1); IR (ATR) ν: 3091, 2959, 2873, 1469, 1427, 1398, 1352, 1260, 1216, 1193, 1116, 1093, 1013, 970, 959, 844, 819, 757, 741, 707, 694 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.66 (t, J = 7.6 Hz, 3H, CH3), 1.48 (sext, J = 7.6 Hz, 2H, CH2), 4.12 (t, J = 7.6 Hz, 2H, CH2), 7.13 (dd, J = 4.8 and 3.6 Hz, 2H, ArH), 7.36 (d, J = 4.8 Hz, 2H, ArH), 7.43 (d, J = 3.6 Hz, 2H, ArH), 7.71 (d, J = 8.4 Hz, 4H, ArH), 7.78 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.7, 124.0, 125.8, 126.2, 126.6, 128.3, 129.4, 136.0, 143.2, 155.3; HRMS m/z calcd for (C25H21N3S2 + H+): 428.1250; found: 428.1241.
4-Propyl-3,5-bis[4-(thiophen-3-yl)phenyl]-4H-1,2,4-triazole (6n). Beige solid in 90% yield, 0.385 g, m.p. 302–305 °C; UV (CH2Cl2) λmax 290.0 nm (ε⋅10−3 44.8 cm−1M−1); IR (ATR) ν: 3094, 2966, 1467, 1423, 1402, 1352, 1206, 1197, 1117, 1089, 1017, 970, 871, 843, 781, 753, 743, 732, 720 cm−1; 1H -NMR (400 MHz, CDCl3): δ 0.66 (t, J = 7.6 Hz, 3H, CH3), 1.48 (sext, J = 7.6 Hz, 2H, CH2), 4.13 (t, J = 7.6 Hz, 2H, CH2), 7.44 (dd, J = 5.2 and 3.2 Hz, 2H, ArH), 7.47 (dd, J = 5.2 and 1.6 Hz, 2H, ArH), 7.58 (dd, J = 3.2 and 1.6 Hz, 2H, ArH), 7.73 (d, J = 8.4 Hz, 4H, ArH), 7.78 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 10.7, 23.5, 46.6, 121.3, 126.1, 126.4, 126.7, 126.8, 129.4, 137.4, 141.2, 155.4; HRMS m/z calcd for (C25H21N3S2 + H+): 428.1250; found: 428.1259.
3,5-Bis(biphenyl-4-yl)-4-butyl-4H-1,2,4-triazole (7a). Creamy solid in 92% yield, 0.396 g, m.p. 297–300 °C; UV (CH2Cl2) λmax 282.0 nm (ε⋅10−3 41.9 cm−1M−1); IR (ATR) ν: 3034, 2953, 2926, 2859, 1472, 1447, 1423, 1384, 1211, 1121, 1098, 1005, 973, 842, 768, 753, 741, 723, 688 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.69 (t, J = 7.6 Hz, 3H, CH3), 1.07 (sext, J = 7.6 Hz, 2H, CH2), 1.45 (quin, J = 7.6 Hz, 2H, CH2), 4.19 (t, J = 7.6 Hz, 2H, CH2), 7.40 (t, J = 7.2 Hz, 2H, ArH), 7.49 (t, J = 7.2 Hz, 4H, ArH), 7.67 (d, J = 7.2 Hz, 4H, ArH), 7.75-7.79 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.0, 44.8, 126.6, 127.1, 127.6, 127.9, 128.9, 129.3, 140.0, 142.8, 155.4; HRMS m/z calcd for (C30H27N3 + H+): 430.2278; found: 430.2277.
4-Butyl-3,5-bis(2′-methylbiphenyl-4-yl)-4H-1,2,4-triazole (7b). Grey solid in 83% yield, 0.380 g, m.p. 182–183 °C; UV (CH2Cl2) λmax 269.0 nm (ε⋅10−3 32.6 cm−1M−1); IR (ATR) ν: 3051, 3015, 2963, 2926, 2859, 1470, 1460, 1421, 1383, 1355, 1326, 1109, 1008, 970, 863, 854, 840, 807, 766, 745, 734, 724 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.70 (t, J = 7.2 Hz, 3H, CH3), 1.10 (sext, J = 7.2 Hz, 2H, CH2), 1.48 (quin, J = 7.2 Hz, 2H, CH2), 2.32 (s, 6H, 2 × CH3), 4.21 (t, J = 7.2 Hz, 2H, CH2), 7.28–7.31 (m, 8H, ArH), 7.50 (d, J = 8.4 Hz, 4H, ArH), 7.74 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 20.4, 32.1, 44.7, 125.9, 126.3, 127.7, 128.7, 129.6, 129.8, 130.5, 135.3, 140.9, 143.8, 155.5; HRMS m/z calcd for (C32H31N3 + H+): 458.2591; found: 458.2588.
4-Butyl-3,5-bis(3′-methylbiphenyl-4-yl)-4H-1,2,4-triazole (7c). White solid in 96% yield, 0.440 g, m.p. 211–213 °C; UV (CH2Cl2) λmax 285.0 nm (ε⋅10−3 44.9 cm−1M−1); IR (ATR) ν: 3020, 2960, 2872, 1607, 1466, 1416, 1340, 1262, 1111, 1094, 1039, 1019, 972, 862, 855, 845, 780, 761, 749, 608 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.69 (t, J = 7.2 Hz, 3H, CH3), 1.06 (sext, J = 7.2 Hz, 2H, CH2), 1.45 (quin, J = 7.2 Hz, 2H, CH2), 2.45 (s, 6H, 2 × CH3), 4.18 (t, J = 7.2 Hz, 2H, CH2), 7.22 (d, J = 7.6 Hz, 2H, ArH), 7.38 (t, J = 7.6 Hz, 2H, ArH), 7.45–7.49 (m, 4H, ArH), 7.75–7.79 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 21.5, 32.1, 44.8, 124.3, 126.6, 127.6, 127.9, 128.7, 128.9, 129.3, 138.6, 140.0, 142.9, 155.4; HRMS m/z calcd for (C32H31N3 + H+): 458.2591; found: 458.2592.
4-Butyl-3,5-bis(2′,6′-dimethylbiphenyl-4-yl)-4H-1,2,4-triazole (7d). White solid in 79% yield, 0.384 g, m.p. 269–271 °C; UV (CH2Cl2) λmax 258.0 nm (ε⋅10-3 31.1 cm−1M−1); IR (ATR) ν: 3035, 2969, 1464, 851, 839, 778, 751 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.66 (t, J = 7.2 Hz, 3H, CH3), 1.07 (sext, J = 7.2 Hz, 2H, CH2), 1.47 (quin, J = 7.2 Hz, 2H, CH2), 2.07 (s, 12H, 4 × CH3), 4.23 (t, J = 7.2 Hz, 2H, CH2), 7.15 (d, J = 7.2 Hz, 4H, ArH), 7.19–7.23 (m, 2H, ArH), 7.34 (d, J = 8.4 Hz, 4H, ArH), 7.76 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.0, 19.1, 20.8, 31.9, 44.5, 126.3, 127.4, 129.1, 129.8, 135.8, 140.8, 143.1, 155.5; HRMS m/z calcd for (C34H35N3 + H+): 486.2904; found: 486.2901.
4-Butyl-3,5-bis(2′-methoxybiphenyl-4-yl)-4H-1,2,4-triazole (7e). White solid in 85% yield, 0.417 g, m.p. 190–193 °C; UV (CH2Cl2) λmax 272.0 nm (ε⋅10−3 29.5 cm−1M−1) and 295.0 (28.3); IR (ATR) ν: 2956, 2932, 2832, 1597, 1582, 1493, 1462, 1423, 1258, 1239, 1122, 1056, 1025, 1005, 974, 866, 837, 801, 756, 736 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.71 (t, J = 7.6 Hz, 3H, CH3), 1.10 (sext, J = 7.6 Hz, 2H, CH2), 1.49 (quin, J = 7.6 Hz, 2H, CH2), 3.85 (s, 6H, 2 × OCH3), 4.20 (t, J = 7.6 Hz, 2H, CH2), 7.02 (d, J = 7.6 Hz, 2H, ArH), 7.07 (t, J = 7.6 Hz, 2H, ArH), 7.35–7.40 (m, 4H, ArH), 7.70 (d, J = 8.4 Hz, 4H, ArH), 7.73 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.4, 32.0, 44.8, 55.6, 111.5, 121.0, 126.3, 128.5, 129.2, 129.7, 130.0, 130.8, 140.3, 155.5, 156.5; HRMS m/z calcd for (C32H31N3O2 + H+): 490.2489; found: 490.2491.
4-Butyl-3,5-bis(3′-methoxybiphenyl-4-yl)-4H-1,2,4-triazole (7f). Grey solid in 82% yield, 0.402 g, m.p. 182–184 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10-3 33.5 cm−1M−1); IR (ATR) ν: 2954, 2930, 2830, 1608, 1583, 1560, 1474, 1421, 1297, 1212, 1172, 1055, 1033, 1015, 856, 839, 779, 752, 693 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.69 (t, J = 7.6 Hz, 3H, CH3), 1.07 (sext, J = 7.6 Hz, 2H, CH2), 1.45 (quin, J = 7.6 Hz, 2H, CH2), 3.90 (s, 6H, 2 × OCH3), 4.19 (t, J = 7.6 Hz, 2H, CH2), 6.95 (dd, J = 8.0 and 2.4 Hz, 2H, ArH), 7.19 (t, J = 2.4 Hz, 2H, ArH), 7.24–7.26 (m, 2H, ArH), 7.40 (t, J = 8.0 Hz, 2H, ArH), 7.74–7.78 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.1, 44.8, 55.4, 112.9, 113.3, 119.6, 126.8, 127.6, 129.3, 130.0, 141.5, 142.6, 155.4, 160.1; HRMS m/z calcd for (C32H31N3O2 + H+): 490.2489; found: 490.2492.
4-Butyl-3,5-bis(3′-nitrobiphenyl-4-yl)-4H-1,2,4-triazole (7g). Creamy solid in 91% yield, 0.473 g, m.p. 252–253 °C; UV (CH2Cl2) λmax 276.0 nm (ε⋅10−3 46.9 cm−1M−1); IR (ATR) ν: 3099, 2926, 2861, 1522, 1490, 1467, 1347, 1292, 1102, 1018, 973, 876, 850, 832, 799, 780, 740, 724, 704 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.71 (t, J = 7.6 Hz, 3H, CH3), 1.09 (sext, J = 7.6 Hz, 2H, CH2), 1.47 (quin, J = 7.6 Hz, 2H, CH2), 4.22 (t, J = 7.6 Hz, 2H, CH2), 7.68 (t, J = 8.0 Hz, 2H, ArH), 7.82 (d, J = 8.4 Hz, 4H, ArH), 7.86 (d, J = 8.4 Hz, 4H, ArH), 8.00 (dd, J = 8.0 and 2.0 Hz, 2H, ArH), 8.27 (dd, J = 8.0 and 2.0 Hz, 2H, ArH), 8.54 (t, J = 2.0 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.1, 45.0, 122.0, 122.7, 127.7, 128.0, 129.7, 130.0, 133.0, 140.3, 141.7, 148.9, 155.1; HRMS m/z calcd for (C30H25N5O4 + H+): 520.1979; found: 520.1967.
3,5-Bis(3′-aminobiphenyl-4-yl)-4-butyl-4H-1,2,4-triazole (7h). Beige solid in 98% yield, 0.451 g, m.p. 244–246 °C; UV (CH2Cl2) λmax 232.0 nm (ε⋅10−3 27.7 cm−1M−1), 258.0 (29.6) and 281.0 (34.1); IR (ATR) ν: 3428, 3348, 2955, 2926, 2871, 1619, 1601, 1588, 1472, 1449, 1422, 1318, 1229, 1172, 973, 836, 777, 755, 744, 665 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.68 (t, J = 7.2 Hz, 3H, CH3), 1.05 (sext, J = 7.2 Hz, 2H, CH2), 1.43 (quin, J = 7.2 Hz, 2H, CH2), 3.81 (br.s, 4H, 2 × NH2), 4.17 (t, J = 7.2 Hz, 2H, CH2), 6.72 (dd, J = 8.0 and 2.0 Hz, 2H, ArH), 6.98 (t, J = 2.0 Hz, 2H, ArH), 7.05 (dd, J = 8.0 and 2.0 Hz, 2H, ArH), 7.26 (t, J = 8.0 Hz, 2H, ArH), 7.71–7.75 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.0, 44.8, 113.7, 114.7, 117.5, 126.6, 127.5, 129.2, 129.9, 141.2, 143.0, 147.0, 155.4; HRMS m/z calcd for (C30H29N5 + H+): 460.2496; found: 460.2479.
4-Butyl-3,5-bis[4-(pyridin-4-yl)phenyl]-4H-1,2,4-triazole (7i). Creamy solid in 92% yield, 0.397 g, m.p. 258–259 °C; UV (CH2Cl2) λmax 281.0 nm (ε⋅10−3 33.7 cm−1M−1); IR (ATR) ν: 2963, 1595, 1540, 1471, 1407, 1217, 971, 857, 811, 771, 757, 736 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.70 (t, J = 7.6 Hz, 3H, CH3), 1.07 (sext, J = 7.6 Hz, 2H, CH2), 1.45 (quin, J = 7.6 Hz, 2H, CH2), 4.21 (t, J = 7.6 Hz, 2H, CH2), 7.58 (d, J = 6.0 Hz, 4H, ArH), 7.81–7.85 (m, 8H, ArH), 8.73 (d, J = 6.0 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.1, 44.9, 121.6, 127.6, 128.4, 129.6, 139.8, 147.1, 150.5, 155.1; HRMS m/z calcd for (C28H25N5 + H+): 432.2183; found: 432.2180.
4-Butyl-3,5-bis[4-(pyridin-3-yl)phenyl]-4H-1,2,4-triazole (7j). White solid in 74% yield, 0.320 g, m.p. 207–209 °C; UV (CH2Cl2) λmax 285.0 nm (ε⋅10−3 40.4 cm−1M−1); IR (ATR) ν: 3033, 2929, 2870, 1575, 1468, 1428, 1413, 1386, 1024, 1000, 972, 853, 803, 774, 746, 710 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.70 (t, J = 7.2 Hz, 3H, CH3), 1.08 (sext, J = 7.2 Hz, 2H, CH2), 1.46 (quin, J = 7.2 Hz, 2H, CH2), 4.21 (t, J = 7.2 Hz, 2H, CH2), 7.42 (dd, J = 7.6 and 4.8 Hz, 2H, ArH), 7.78 (d, J = 8.4 Hz, 4H, ArH), 7.84 (d, J = 8.4 Hz, 4H, ArH), 7.95 (dt, J = 7.6 and 1.6 Hz, 2H, ArH), 8.66 (dd, J = 4.8 and 1.6 Hz, 2H, ArH), 8.93 (d, J = 1.6 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.1, 44.9, 123.7, 127.5, 127.6, 129.6, 134.4, 135.5, 139.5, 148.3, 149.1, 155.2; HRMS m/z calcd for (C28H25N5 + H+): 432.2183; found: 432.2181.
4-Butyl-3,5-bis[4-(furan-2-yl)phenyl]-4H-1,2,4-triazole (7k). Creamy solid in 75% yield, 0.307 g, m.p. 264–267 °C; UV (CH2Cl2) λmax 310.0 nm (ε⋅10-3 43.6 cm−1M−1); IR (ATR) ν: 2956, 2926, 2871, 1615, 1491, 1471, 1220, 1189, 1157, 1115, 1008, 972, 904, 884, 848, 805, 773, 735, 702 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.66 (t, J = 7.6 Hz, 3H, CH3), 1.03 (sext, J = 7.6 Hz, 2H, CH2), 1.40 (quin, J = 7.6 Hz, 2H, CH2), 4.14 (t, J = 7.6 Hz, 2H, CH2), 6.53 (dd, J = 3.2 and 1.6 Hz, 2H, ArH), 6.78 (d, J = 3.2 Hz, 2H, ArH), 7.53 (d, J = 1.6 Hz, 2H, ArH), 7.71 (d, J = 8.4 Hz, 4H, ArH), 7.83 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.0, 44.8, 106.4, 111.9, 124.1, 126.4, 129.2, 132.3, 142.8, 153.0, 155.4; HRMS m/z calcd for (C26H23N3O2 + H+): 410.1863; found: 410.1866.
4-Butyl-3,5-bis[4-(furan-3-yl)phenyl]-4H-1,2,4-triazole (7l). Yellow solid in 94% yield, 0.384 g, m.p. 274–277 °C; UV (CH2Cl2) λmax 285.0 nm (ε⋅10−3 37.3 cm−1M−1); IR (ATR) ν: 3134, 2960, 1507, 1469, 1194, 1162, 1115, 1094, 1054, 1015, 975, 923, 873, 842, 785, 749 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.67 (t, J = 7.6 Hz, 3H, CH3), 1.04 (sext, J = 7.6 Hz, 2H, CH2), 1.41 (quin, J = 7.6 Hz, 2H, CH2), 4.14 (t, J = 7.6 Hz, 2H, CH2), 6.77 (d, J = 1.6 Hz, 2H, ArH), 7.53 (d, J = 1.6 Hz, 2H, ArH), 7.65 (d, J = 8.8 Hz, 4H, ArH), 7.71 (d, J = 8.8 Hz, 4H, ArH), 7.83 (s, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.0, 44.8, 108.6, 125.7, 126.2, 126.3, 129.4, 134.2, 139.2, 144.0, 155.4; HRMS m/z calcd for (C26H23N3O2 + H+): 410.1863; found: 410.1867.
4-Butyl-3,5-bis[4-(thiophen-2-yl)phenyl]-4H-1,2,4-triazole (7m). Grey solid in 49% yield, 0.216 g, m.p. 289–292 °C; UV (CH2Cl2) λmax 312.0 nm (ε⋅10−3 37.4 cm−1M−1); IR (ATR) ν: 2955, 1472, 1426, 1400, 1215, 1193, 1115, 1099, 974, 844, 819, 742, 695 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.68 (t, J = 7.6 Hz, 3H, CH3), 1.05 (sext, J = 7.6 Hz, 2H, CH2), 1.43 (quin, J = 7.6 Hz, 2H, CH2), 4.15 (t, J = 7.6 Hz, 2H, CH2), 7.13 (dd, J = 4.8 and 3.6 Hz, 2H, ArH), 7.36 (d, J = 4.8 Hz, 2H, ArH), 7.43 (d, J = 3.6 Hz, 2H, ArH), 7.70 (d, J = 8.4 Hz, 4H, ArH), 7.78 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.0, 44.9, 124.0, 125.8, 126.2, 126.6, 128.3, 129.4, 136.0, 143.2, 155.3; HRMS m/z calcd for (C26H23N3S2 + H+): 442.1406; found: 442.1412.
4-Butyl-3,5-bis[4-(thiophen-3-yl)phenyl]-4H-1,2,4-triazole (7n). Beige solid in 77% yield, 0.342 g, m.p. 288–290 °C; UV (CH2Cl2) λmax 289.0 nm (ε⋅10−3 37.4 cm−1M−1); IR (ATR) ν: 3067, 2955, 2872, 1470, 1428, 1363, 1196, 1094, 1014, 973, 867, 841, 782, 746, 688 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.68 (t, J = 7.6 Hz, 3H, CH3), 1.04 (sext, J = 7.6 Hz, 2H, CH2), 1.41 (quin, J = 7.6 Hz, 2H, CH2), 4.13 (t, J = 7.6 Hz, 2H, CH2), 7.43–7.47 (m, 2H, ArH), 7.56–7,59 (m, 2H, ArH), 7.68–7.78 (m, 10H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.2, 19.3, 32.0, 44.8, 121.4, 126.1, 126.2, 126.7, 126.8, 129.4, 132.3, 141.2, 154.6; HRMS m/z calcd for (C26H23N3S2 + H+): 442.1406; found: 442.1397.
3,5-Bis(biphenyl-4-yl)-4-hexyl-4H-1,2,4-triazole (8a). White solid in 92% yield, 0.421 g, m.p. 240–242 °C; UV (CH2Cl2) λmax 282.0 nm (ε⋅10-3 45.4 cm−1M−1); IR (ATR) ν: 3028, 2926, 2855, 1474, 1444, 1429, 1379, 1072, 1007, 967, 858, 847, 835, 767, 738, 695 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.6 Hz, 3H, CH3), 1.00–1.11 (m, 6H, 3 × CH2), 1.46 (quin, J = 7.6 Hz, 2H, CH2), 4.18 (t, J = 7.6 Hz, 2H, CH2), 7.40 (t, J = 7.2 Hz, 2H, ArH), 7.49 (t, J = 7.2 Hz, 4H, ArH), 7.67 (d, J = 7.2 Hz, 4H, ArH), 7.75–7.79 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 29.9, 30.7, 45.0, 126.7, 127.1, 127.6, 127.9, 128.9, 129.3, 140.1, 142.8, 155.4; HRMS m/z calcd for (C32H31N3 + H+): 458.2591; found: 458.2588.
4-Hexyl-3,5-bis(2′-methylbiphenyl-4-yl)-4H-1,2,4-triazole (8b). White solid in 72% yield, 0.350 g, m.p. 185–186 °C; UV (CH2Cl2) λmax 269.0 nm (ε⋅10−3 31.5 cm−1M−1); IR (ATR) ν: 3022, 2955, 2929, 2857, 1470, 1425, 1378, 1261, 1099, 1007, 966, 840, 766, 743, 723 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.75 (t, J = 7.2 Hz, 3H, CH3), 1.01–1.13 (m, 6H, 3 × CH2), 1.49 (quin, J = 7.2 Hz, 2H, CH2), 2.32 (s, 6H, 2 × CH3), 4.20 (t, J = 7.6 Hz, 2H, CH2), 7.27–7.32 (m, 8H, ArH), 7.50 (d, J = 8.4 Hz, 4H, ArH), 7.75 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 20.4, 22.1, 25.6, 29.9, 30.7, 44.9, 125.9, 126.3, 127.7, 128.7, 129.6, 129.8, 130.5, 135.3, 140.9, 143.8, 155.5; HRMS m/z calcd for (C34H35N3 + H+): 486.2904; found: 486.2905.
4-Hexyl-3,5-bis(3′-methylbiphenyl-4-yl)-4H-1,2,4-triazole (8c). White solid in 91% yield, 0.442 g, m.p. 217–218 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 40.3 cm−1M−1); IR (ATR) ν: 3017, 2929, 2863, 1607, 1468, 1416, 1380, 1338, 1112, 1017, 968, 855, 843, 754, 691 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 1.01–1.11 (m, 6H, 3 × CH2), 1.45 (quin, J = 7.2 Hz, 2H, CH2), 2.45 (s, 6H, 2 × CH3), 4.17 (t, J = 7.6 Hz, 2H, CH2), 7.22 (d, J = 7.6 Hz, 2H, ArH), 7.37 (t, J = 7.6 Hz, 2H, ArH), 7.45–7.48 (m, 4H, ArH), 7.74–7.77 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 21.5, 22.2, 25.7, 29.9, 30.7, 45.0, 124.2, 126.6, 127.6, 127.9, 128.7, 128.8, 129.2, 138.6, 140.1, 142.9, 155.4; HRMS m/z calcd for (C34H35N3 + H+): 486.2904; found: 486.2902.
4-Hexyl-3,5-bis(2′,6′-dimethylbiphenyl-4-yl)-4H-1,2,4-triazole (8d). White solid in 93% yield, 0.478 g, m.p. 200–202 °C; UV (CH2Cl2) λmax 259.0 nm (ε⋅10−3 26.8 cm−1M−1); IR (ATR) ν: 3014, 2954, 2930, 2855, 1464, 1418, 1380, 1260, 1096, 1005, 969, 852, 839, 770, 737 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.75 (t, J = 7.2 Hz, 3H, CH3), 1.00–1.13 (m, 6H, 3 × CH2), 1.48 (quin, J = 7.2 Hz, 2H, CH2), 2.07 (s, 12H, 4 × CH3), 4.22 (t, J = 7.6 Hz, 2H, CH2), 7.14 (d, J = 7.2 Hz, 4H, ArH), 7.19–7.23 (m, 2H, ArH), 7.34 (d, J = 8.4 Hz, 4H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.7, 20.8, 22.1, 25.6, 29.7, 30.6, 44.9, 115.6, 126.3, 127.4, 129.1, 129.8, 135.8, 140.8, 143.1, 155.6; HRMS m/z calcd for (C36H39N3 + H+): 514.3217; found: 514.3217.
4-Hexyl-3,5-bis(2′-methoxybiphenyl-4-yl)-4H-1,2,4-triazole (8e). Creamy solid in 88% yield, 0.456 g, m.p. 171–172 °C; UV (CH2Cl2) λmax 273.0 nm (ε⋅10−3 28.1 cm−1M−1) and 296.0 (28.8); IR (ATR) ν: 3049, 2953, 2860, 1599, 1494, 1467, 1433, 1421, 1257, 1235, 1179, 1125, 1112, 1029, 1016, 1005, 861, 841, 801, 748, 737, 721 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.76 (t, J = 7.2 Hz, 3H, CH3), 1.02–1.13 (m, 6H, 3 × CH2), 1.50 (quin, J = 7.2 Hz, 2H, CH2), 3.85 (s, 6H, 2 × OCH3), 4.20 (t, J = 7.6 Hz, 2H, CH2), 7.03 (d, J = 8.4 Hz, 2H, ArH), 7.07 (t, J = 7.6 Hz, 2H, ArH), 7.34–7.40 (m, 4H, ArH), 7.70 (d, J = 8.4 Hz, 4H, ArH), 7.73 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 30.0, 30.7, 44.9, 55.6, 111.5, 121.0, 126.3, 128.5, 129.2, 129.7, 130.0, 130.8, 140.3, 155.5, 156.5; HRMS m/z calcd for (C34H35N3O2 + H+): 518.2802; found: 518.2800.
4-Hexyl-3,5-bis(3′-methoxybiphenyl-4-yl)-4H-1,2,4-triazole (8f). White solid in 83% yield, 0.430 g, m.p. 182–184 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 33.6 cm−1M−1); IR (ATR) ν: 3036, 2954, 2931, 2853, 1599, 1590, 1476, 1461, 1428, 1414, 1323, 1300, 1221, 1177, 1049, 1035, 1022, 861, 834, 788, 747 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 1.01–1.10 (m, 6H, 3 × CH2), 1.46 (quin, J = 7.2 Hz, 2H, CH2), 3.90 (s, 6H, 2 × OCH3), 4.18 (t, J = 7.2 Hz, 2H, CH2), 6.95 (dd, J = 8.0 and 2.4 Hz, 2H, ArH), 7.19 (d, J = 2.4 Hz, 2H, ArH), 7.23–7.27 (m, 2H, ArH), 7.40 (t, J = 8.0 Hz, 2H, ArH), 7.74–7.78 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 29.9, 30.7, 45.0, 55.4, 113.0, 113.3, 119.6, 126.9, 127.6, 129.3, 130.0, 141.6, 142.6, 155.4, 160.1; HRMS m/z calcd for (C34H35N3O2 + H+): 518.2802; found: 518.2801.
4-Hexyl-3,5-bis(3′-nitrobiphenyl-4-yl)-4H-1,2,4-triazole (8g). Creamy solid in 85% yield, 0.466 g, m.p. 223–225 °C; UV (CH2Cl2) λmax 276.0 nm (ε⋅10-3 48.3 cm−1M−1); IR (ATR) ν: 3086, 2927, 2857, 1522, 1472, 1344, 1294, 1105, 1085, 968, 877, 843, 813, 778, 730, 702, 684 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.74 (t, J = 7.2 Hz, 3H, CH3), 1.01–1.13 (m, 6H, 3 × CH2), 1.47 (quin, J = 7.6 Hz, 2H, CH2), 4.21 (t, J = 7.6 Hz, 2H, CH2), 7.68 (t, J = 7.6 Hz, 2H, ArH), 7.82 (d, J = 8.4 Hz, 4H, ArH), 7.86 (d, J = 8.4 Hz, 4H, ArH), 8.00 (dd, J = 7.6 and 2.0 Hz, 2H, ArH), 8.27 (dd, J = 7.6 and 2.0 Hz, 2H, ArH), 8.53 (t, J = 2.0 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 30.0, 30.7, 45.1, 122.0, 122.7, 127.7, 128.0, 129.7, 130.0, 133.0, 140.3, 141.7, 148.8, 155.1; HRMS m/z calcd for (C32H29N5O4 + H+): 548.2292; found: 548.2299.
3,5-Bis(3′-aminobiphenyl-4-yl)-4-hexyl-4H-1,2,4-triazole (8h). Beige solid in 78% yield, 0.381 g, m.p. 181–183 °C; UV (CH2Cl2) λmax 233.0 nm (ε⋅10−3 30.3 cm−1M−1), 258.0 (32.8) and 281.0 (37.7); IR (ATR) ν: 3442, 3369, 3200, 3034, 2956, 2926, 2856, 1617, 1601, 1586, 1562, 1474, 1427, 1321, 1227, 1166, 993, 888, 835, 780, 746, 722 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 0.98–1.10 (m, 6H, 3 × CH2), 1.44 (quin, J = 7.2 Hz, 2H, CH2), 3.78 (br.s, 4H, 2 × NH2), 4.16 (t, J = 7.6 Hz, 2H, CH2), 6.73 (dd, J = 7.6 and 2.0 Hz, 2H, ArH), 6.98 (t, J = 2.0 Hz, 2H, ArH), 7.05 (dd, J = 7.6 and 2.0 Hz, 2H, ArH), 7.26 (t, J = 7.6 Hz, 2H, ArH), 7.72–7.74 (m, 8H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.6, 29.9, 30.7, 45.0, 113.7, 114.7, 117.5, 126.6, 127.5, 129.2, 129.9, 141.2, 143.0, 146.9, 155.4; HRMS m/z calcd for (C32H33N5 + H+): 488.2809; found: 488.2817.
4-Hexyl-3,5-bis[4-(pyridin-4-yl)phenyl]-4H-1,2,4-triazole (8i). White solid in 86% yield, 0.396 g, m.p. 205–207 °C; UV (CH2Cl2) λmax 281.0 nm (ε⋅10−3 46.6 cm−1M−1); IR (ATR) ν: 2924, 2855, 1595, 1540, 1471, 1408, 1216, 993, 967, 857, 812, 771, 753, 737, 667 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 0.99-1.11 (m, 6H, 3 × CH2), 1.46 (quin, J = 7.2 Hz, 2H, CH2), 4.20 (t, J = 7.2 Hz, 2H, CH2), 7.58 (d, J = 6.0 Hz, 4H, ArH), 7.82–7.85 (m, 8H, ArH), 8.73 (d, J = 6.0 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.1, 25.6, 29.9, 30.7, 45.1, 121.6, 127.6, 128.4, 129.6, 139.8, 147.1, 150.5, 155.1; HRMS m/z calcd for (C30H29N5 + H+): 460.2496; found: 460.2499.
4-Hexyl-3,5-bis[4-(pyridin-3-yl)phenyl]-4H-1,2,4-triazole (8j). Creamy solid in 83% yield, 0.382 g, m.p. 198–199 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 41.6 cm−1M−1); IR (ATR) ν: 2927, 2855, 1574, 1469, 1415, 1380, 1102, 1026, 1001, 967, 849, 839, 804, 751, 709 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 1.00–1.11 (m, 6H, 3 × CH2), 1.47 (quin, J = 7.2 Hz, 2H, CH2), 4.20 (t, J = 7.2 Hz, 2H, CH2), 7.42 (dd, J = 8.0 and 4.8 Hz, 2H, ArH), 7.78 (d, J = 8.4 Hz, 4H, ArH), 7.83 (d, J = 8.4 Hz, 4H, ArH), 7.96 (dt, J = 8.0 and 1.6 Hz, 2H, ArH), 8.66 (dd, J = 4.8 and 1.6 Hz, 2H, ArH), 8.94 (d, J = 1.6 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 29.9, 30.7, 45.1, 123.7, 127.5, 127.7, 129.6, 134.4, 135.5, 139.5, 148.3, 149.1, 155.2; HRMS m/z calcd for (C30H29N5 + H+): 460.2496; found: 460.2497.
3,5-Bis[4-(furan-2-yl)phenyl]-4-hexyl-4H-1,2,4-triazole (8k). Creamy solid in 89% yield, 0.390 g, m.p. 199–202 °C; UV (CH2Cl2) λmax 310.0 nm (ε⋅10−3 37.8 cm−1M−1); IR (ATR) ν: 2933, 1687, 1611, 1473, 1374, 1259, 1180, 1092, 1009, 903, 844, 800, 731, 664 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.72 (t, J = 7.2 Hz, 3H, CH3), 0.97–1.10 (m, 6H, 3 × CH2), 1.41 (quin, J = 7.2 Hz, 2H, CH2), 4.13 (t, J = 7.6 Hz, 2H, CH2), 6.53 (dd, J = 3.6 and 2.0 Hz, 2H, ArH), 6.78 (d, J = 3.6 Hz, 2H, ArH), 7.53 (d, J = 2.0 Hz, 2H, ArH), 7.70 (d, J = 8.8 Hz, 4H, ArH), 7.83 (d, J = 8.8 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.7, 22.2, 25.7, 29.8, 30.7, 45.0, 106.4, 111.9, 124.1, 126.3, 129.2, 132.3, 142.8, 153.0, 155.3; HRMS m/z calcd for (C28H27N3O2 + H+): 438.2176; found: 438.2175.
3,5-Bis[4-(furan-3-yl)phenyl]-4-hexyl-4H-1,2,4-triazole (8l). Yellow solid in 69% yield, 0.302 g, m.p. 175–178 °C; UV (CH2Cl2) λmax 284.0 nm (ε⋅10−3 17.8 cm−1M−1); IR (ATR) ν: 3093, 2933, 2864, 1740, 1615, 1475, 1429, 1261, 1164, 1116, 1055, 1015, 957, 922, 873, 839, 785, 750, 723, 695 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 0.97–1.10 (m, 6H, 3 × CH2), 1.41 (quin, J = 7.2 Hz, 2H, CH2), 4.13 (t, J = 7.6 Hz, 2H, CH2), 6.77 (dd, J = 2.0 and 1.2 Hz, 2H, ArH), 7.53 (d, J = 2.0 Hz, 2H, ArH), 7.65 (d, J = 8.8 Hz, 4H, ArH), 7.69 (d, J = 8.8 Hz, 4H, ArH), 7.83 (d, J = 1.2 Hz, 2H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 29.8, 30.7, 45.0, 108.6, 125.7, 126.1, 126.2, 129.3, 134.2, 139.2, 144.0, 155.3; HRMS m/z calcd for (C28H27N3O2 + H+): 438.2176; found: 438.2173.
4-Hexyl-3,5-bis[4-(thiophen-2-yl)phenyl]-4H-1,2,4-triazole (8m). Beige solid in 37% yield, 0.174 g, m.p. 219–220 °C; UV (CH2Cl2) λmax 312.0 nm (ε⋅10−3 42.0 cm−1M−1); IR (ATR) ν: 2954, 2923, 2853, 1611, 1471, 1427, 1400, 1377, 1351, 1259, 1213, 1115, 959, 848, 818, 776, 752, 662 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 0.99–1.09 (m, 6H, 3 × CH2), 1.43 (quin, J = 7.2 Hz, 2H, CH2), 4.14 (t, J = 7.2 Hz, 2H, CH2), 7.13 (dd, J = 4.8 and 3.6 Hz, 2H, ArH), 7.36 (d, J = 4.8 Hz, 2H, ArH), 7.43 (d, J = 3.6 Hz, 2H, ArH), 7.71 (d, J = 8.4 Hz, 4H, ArH), 7.78 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 29.9, 30.7, 45.0, 124.0, 125.8, 126.2, 126.6, 128.3, 129.4, 136.0, 143.2, 155.3; HRMS m/z calcd for (C28H27N3S2 + H+): 470.1719; found: 470.1707.
4-Hexyl-3,5-bis[4-(thiophen-3-yl)phenyl]-4H-1,2,4-triazole (8n). Creamy solid in 62% yield, 0.291 g, m.p. 237–239 °C; UV (CH2Cl2) λmax 290.0 nm (ε⋅10−3 39.6 cm−1M−1); IR (ATR) ν: 3065, 2925, 2856, 1614, 1467, 1426, 1355, 1260, 1194, 1100, 1014, 864, 846, 781, 749 cm−1; 1H-NMR (400 MHz, CDCl3): δ 0.73 (t, J = 7.2 Hz, 3H, CH3), 0.98–1.09 (m, 6H, 3 × CH2), 1.43 (quin, J = 7.2 Hz, 2H, CH2), 4.15 (t, J = 7.2 Hz, 2H, CH2), 7.44 (dd, J = 5.2 and 2.8 Hz, 2H, ArH), 7.47 (dd, J = 5.2 and 1.6 Hz, 2H, ArH), 7.57 (dd, J = 2.8 and 1.6 Hz, 2H, ArH), 7.72 (d, J = 8.4 Hz, 4H, ArH), 7.77 (d, J = 8.4 Hz, 4H, ArH); 13C-NMR (100 MHz, CDCl3): δ 13.8, 22.2, 25.7, 29.9, 30.7, 45.0, 121.3, 126.1, 126.4, 126.7, 126.8, 129.4, 137.3, 141.2, 155.4; HRMS m/z calcd for (C28H27N3S2 + H+): 470.1719; found: 470.1728.