In Vitro Fecal Fermentation of High Pressure-Treated Fruit Peels Used as Dietary Fiber Sources
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Fruit Peels
2.2. In Vitro Fecal Fermentation of Fruit Peels
2.2.1. Total Gas Production and pH Changes
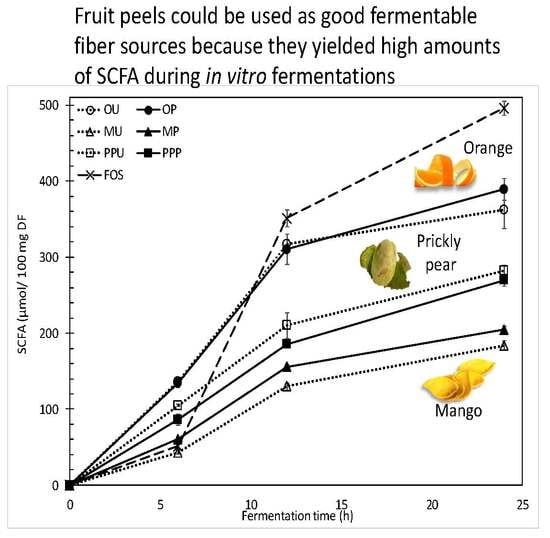
2.2.2. SCFA Production and Profiles
3. Materials and Methods
3.1. Dietary Fiber Substrates
3.2. Chemical Characterization
3.3. Upper Gastrointestinal (GI) Digestion
3.4. In Vitro Fecal Fermentation
3.5. In Vitro Fermentation Products
3.5.1. Total Gas Production and pH Changes Measurements
3.5.2. SCFA Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kale, M.S.; Pai, D.A.; Hamaker, B.R.; Campanella, O.H. Structure- function relationships for corn bran arabinoxylans. J. Cereal Sci. 2010, 52, 368–372. [Google Scholar] [CrossRef]
- Rumpagaporn, P.; Reuhs, B.L.; Kaur, A.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Structural features of soluble cereal arabinoxylan fibers associated with a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr. Polym. 2015, 130, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.E.; Nakatsu, C.H.; Kazem, A.E.; Arioglu-Tuncil, S.; Reuhs, B.; Martens, E.C.; Hamaker, B.R. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J. Funct. Foods 2017, 32, 347–357. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D.V.; de Vrese, M.; Açil, Y.; Schrezenmeir, J. Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats—impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. NFS J. 2016, 3, 41–50. [Google Scholar] [CrossRef]
- Shanahan, F. Probiotics in inflammatory bowel disease–Therapeutic rationale and role. Adv. Drug Deliv. Rev. 2004, 56, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; Patterson, J.A.; Hamaker, B.R. Structural differences among alkali-soluble arabinoxylans from maize (Zea mays), rice (Oryza sativa), and wheat (Triticum aestivum) brans influence human fecal fermentation profiles. J. Agric. Food Chem. 2010, 58, 493–499. [Google Scholar] [CrossRef]
- Kaur, A.; Rose, D.J.; Rumpagaporn, P.; Patterson, J.A.; Hamaker, B.R. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J. Food Sci. 2011, 76, 137–142. [Google Scholar] [CrossRef]
- Kittisuban, P.; Lee, B.H.; Suphantharika, M.; Hamaker, B.R. Slow glucose release property of enzyme-synthesized highly branched maltodextrins differs among starch sources. Carbohydr. Polym. 2014, 107, 182–191. [Google Scholar] [CrossRef]
- Rose, D.J.; DeMeo, M.T.; Keshavarzian, A.; Hamaker, B.R. Influence of dietary fiber on inflammatory bowel disease and colon cancer: Importance of fermentation pattern. Nutr. Rev. 2007, 65, 51–62. [Google Scholar] [CrossRef]
- Sands, A.L.; Leidy, H.J.; Hamaker, B.R.; Maguire, P.; Campbell, W.W. Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans. Nutr. Res. 2009, 29, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Serna-Saldívar, S.O.; Welti-Chanes, J. Dietary fiber concentrates from fruit and vegetable by-products: processing, modification, and application as functional ingredients. Food Bioprocess Technol. 2018, 11, 1439–1463. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. The dietary fiber profile of fruit peels and functionality modifications induced by high hydrostatic pressure treatments. Food Sci. Technol. Int. 2017, 23, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Campanella, O.H.; Welti-Chanes, J. Influence of drying method on the composition, physicochemical properties and prebiotic potential of dietary fibre concentrates from fruit peels. J. Food Qual. 2018, 1–11. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Advances in the functional characterization and extraction processes of dietary fiber. Food Eng. Rev. 2015, 8, 251–271. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Martín-Belloso, O.; Welti-Chanes, J. High hydrostatic pressure and mild heat treatments for the modification of orange peel dietary fiber: Effects on hygroscopic properties and functionality. Food Bioprocess Technol. 2018, 11, 110–121. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Habibi, Y.; Heyraud, A.; Mahrouz, M.; Vignon, M.R. Structural features of pectic polysaccharides from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1119–1127. [Google Scholar] [CrossRef]
- Habibi, Y.; Heux, L.; Mahrouz, M.; Vignon, M. Morphological and structural study of seed pericarp of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2008, 72, 102–112. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Marais, M.; Vignon, M. An arabinogalactan from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1201–1205. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S. The effect of extrusion processing on the physiochemical properties of extruded orange pomace. Food Chem. 2016, 192, 363–369. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Queirós, R.; Rocha-Santos, T.A.P.; Saraiva, J.A.; Gomes, A.M.P.; Duarte, A.C. Bioactive polysaccharides extracts from Sargassum muticum by high hydrostatic pressure. J. Food Process. Preserv. 2016, 1–12. [Google Scholar]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Rupérez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Rose, D.J.; Keshavarzian, A.; Patterson, J.A.; Venkatachalam, M.; Gillevet, P.; Hamaker, B.R. Starch-entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol. Nutr. Food Res. 2009, 53, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Costabile, A.; Martin-Pelaez, S.; Vitaglione, P.; Klinder, A.; Gibson, G.R.; Fogliano, V. Potential prebiotic activity of oligosaccharides obtained by enzymatic conversion of durum wheat insoluble dietary fibre into soluble dietary fibre. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lebet, V.; Arrigoni, E.; Amadò, R. Measurement of fermentation products and substrate disappearance during incubation of dietary fibre sources with human faecal flora. LWT-Food Sci. Technol. 1998, 31, 473–479. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Li, L.; Gibson, G.R.; Sanz, M.L.; Rastall, R.A. In vitro fermentation by human fecal microflora of wheat arabinoxylans. J. Agric. Food Chem. 2007, 55, 4589–4595. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Wang, T.; Sun, X.; Raddatz, J.; Chen, G. Effects of microfluidization on microstructure and physicochemical properties of corn bran. J. Cereal Sci. 2013, 58, 355–361. [Google Scholar] [CrossRef]
- Wang, T.; Sun, X.; Zhou, Z.; Chen, G. Effects of microfluidization process on physicochemical properties of wheat bran. Food Res. Int. 2012, 48, 742–747. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [PubMed]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- York, W.S.; Darvill, A.G.; McNeil, M.; Stevenson, T.T.; Albersheim, P. Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 1986, 118, 3–40. [Google Scholar]
- Lebet, V.; Arrigoni, E.; Amadò, R. Digestion procedure using mammalian enzymes to obtain substrates for in vitro fermentation studies. LWT-Food Sci. Technol. 1998, 509–515. [Google Scholar] [CrossRef]
- Rose, D.J.; Venema, K.; Keshavarzian, A.; Hamaker, B.R. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br. J. Nutr. 2010, 103, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Orange | Mango | Prickly pear | ||||
|---|---|---|---|---|---|---|
| OU | OP | MU | MP | PPU | PPP | |
| Rha | 5.71 ± 0.25 a | 6.22 ± 0.02 a | 2.01 ± 0.22 b | 2.63 ± 0.02 a | 4.22 ± 0.96 b | 7.61 ± 0.25 a |
| Fuc | 3.15 ± 0.04 a | 2.40 ± 0.09 b | 2.01 ± 0.16 b | 2.71 ± 0.10 a | 3.56 ± 0.38 a | 3.39 ± 0.19 a |
| Ara | 35.12 ± 1.51 a | 35.73 ± 0.47 a | 33.17 ± 1.61 a | 34.34 ± 1.94 a | 30.80 ± 1.09 a | 22.60 ± 0.58 b |
| Xyl | 7.26 ± 0.27 a | 7.48 ± 0.26 a | 5.76 ± 0.20 b | 6.60 ± 0.37 a | 12.85 ± 0.54 b | 14.24 ± 0.25 a |
| Man | 5.06 ± 0.03 a | 3.81 ± 0.25 b | 2.01 ± 0.09 b | 2.99 ± 0.37 a | 4.33 ± 0.04 a | 3.82 ± 0.23 a |
| Gal | 25.26 ± 0.84 a | 25.97 ± 0.63 a | 32.43 ± 0.83 b | 34.15 ± 1.35 a | 25.27 ± 2.01 a | 24.40 ± 1.74 a |
| Glu | 19.44 ± 0.71 a | 17.28 ± 0.41 b | 21.63 ± 1.75 a | 18.08 ± 0.17 b | 21.91 ± 1.61 a | 24.13 ± 0.98 a |
| Orange | Mango | Prickly Pear | FOS | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OU | OP | MU | MP | PPU | PPP | |||||||||||||||||||||||
| Acetate | ||||||||||||||||||||||||||||
| 6 h | 119.3 | ± | 2.7 | a,1 | 117.2 | ± | 5.0 | a,1 | 43.3 | ± | 2.5 | a,1 | 54.2 | ± | 3.9 | b,1 | 86.6 | ± | 4.6 | a,1 | 72.9 | ± | 4.7 | a,1 | 45.5 | ± | 4.4 | 1 |
| 12 h | 243.4 | ± | 4.5 | a,2 | 239.7 | ± | 14.8 | a,2 | 101.6 | ± | 1.9 | a,2 | 121.9 | ± | 2.6 | b,2 | 168.1 | ± | 12.6 | a,2 | 149.4 | ± | 2.6 | a,2 | 273.8 | ± | 8.6 | 2 |
| 24 h | 274.7 | ± | 18.2 | a,3 | 296.3 | ± | 10.4 | a,3 | 143.0 | ± | 5.5 | a,3 | 161.0 | ± | 3.7 | b,3 | 219.4 | ± | 5.3 | a,3 | 211.0 | ± | 7.9 | a,3 | 336.5 | ± | 4.0 | 3 |
| Propionate | ||||||||||||||||||||||||||||
| 6 h | 11.4 | ± | 1.1 | a,1 | 11.6 | ± | 0.5 | a,1 | 0.0 | ± | 0.0 | a,1 | 0.0 | ± | 0.0 | a,1 | 12.3 | ± | 1.9 | a,1 | 10.4 | ± | 0.7 | a,1 | 0.0 | ± | 0.0 | 1 |
| 12 h | 42.4 | ± | 0.9 | a,2 | 41.4 | ± | 2.9 | a,2 | 21.3 | ± | 1.3 | a,2 | 25.0 | ± | 0.4 | b,2 | 29.8 | ± | 3.1 | a,2 | 26.0 | ± | 0.4 | a,2 | 48.6 | ± | 1.8 | 2 |
| 24 h | 46.4 | ± | 3.5 | a,2 | 49.6 | ± | 1.6 | a,3 | 27.9 | ± | 0.3 | a,3 | 29.4 | ± | 0.5 | b,3 | 38.3 | ± | 1.0 | a,3 | 36.8 | ± | 0.5 | a,3 | 110.5 | ± | 4.1 | 3 |
| Butyrate | ||||||||||||||||||||||||||||
| 6 h | 5.6 | ± | 0.9 | a,1 | 5.8 | ± | 0.4 | a,1 | 0.0 | ± | 0.0 | a,1 | 4.0 | ± | 0.1 | b,1 | 5.5 | ± | 0.9 | a,1 | 3.7 | ± | 0.0 | a,1 | 3.4 | ± | 0.2 | 1 |
| 12 h | 31.3 | ± | 0.9 | a,2 | 29.4 | ± | 2.7 | a,2 | 7.0 | ± | 1.0 | a,2 | 8.9 | ± | 0.4 | b,2 | 12.1 | ± | 1.6 | a,2 | 10.1 | ± | 0.2 | a,2 | 28.9 | ± | 0.9 | 2 |
| 24 h | 41.2 | ± | 3.3 | a,3 | 43.5 | ± | 2.4 | a,3 | 12.6 | ± | 0.3 | a,3 | 14.1 | ± | 0.7 | b,3 | 24.2 | ± | 1.0 | a,3 | 21.9 | ± | 0.7 | a,3 | 49.1 | ± | 1.8 | 3 |
| Fruit Peel | Treatment | Nomenclature |
|---|---|---|
| Orange | Untreated | OU |
| 600 MPa/55 °C/20 min | OP | |
| Mango | Untreated | MU |
| 600 MPa/22 °C/10 min | MP | |
| Prickly pear | Untreated | PPU |
| 600 MPa/55 °C/10 min | PPP |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Kazem, A.E.; Campanella, O.H.; Cano, M.P.; Hamaker, B.R.; Serna-Saldívar, S.O.; Welti-Chanes, J. In Vitro Fecal Fermentation of High Pressure-Treated Fruit Peels Used as Dietary Fiber Sources. Molecules 2019, 24, 697. https://doi.org/10.3390/molecules24040697
Tejada-Ortigoza V, Garcia-Amezquita LE, Kazem AE, Campanella OH, Cano MP, Hamaker BR, Serna-Saldívar SO, Welti-Chanes J. In Vitro Fecal Fermentation of High Pressure-Treated Fruit Peels Used as Dietary Fiber Sources. Molecules. 2019; 24(4):697. https://doi.org/10.3390/molecules24040697
Chicago/Turabian StyleTejada-Ortigoza, Viridiana, Luis Eduardo Garcia-Amezquita, Ahmad E. Kazem, Osvaldo H. Campanella, M. Pilar Cano, Bruce R. Hamaker, Sergio O. Serna-Saldívar, and Jorge Welti-Chanes. 2019. "In Vitro Fecal Fermentation of High Pressure-Treated Fruit Peels Used as Dietary Fiber Sources" Molecules 24, no. 4: 697. https://doi.org/10.3390/molecules24040697
APA StyleTejada-Ortigoza, V., Garcia-Amezquita, L. E., Kazem, A. E., Campanella, O. H., Cano, M. P., Hamaker, B. R., Serna-Saldívar, S. O., & Welti-Chanes, J. (2019). In Vitro Fecal Fermentation of High Pressure-Treated Fruit Peels Used as Dietary Fiber Sources. Molecules, 24(4), 697. https://doi.org/10.3390/molecules24040697