Micrometric Wrinkled Patterns Spontaneously Formed on Hydrogel Thin Films via Argon Plasma Exposure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Functionalization Effect on Wrinkle Hydrogel Film Adherence
2.1.1. Layer Thickness and Contact Angle
2.1.2. TSM Concentration Effect on Wrinkled Pattern Morphology
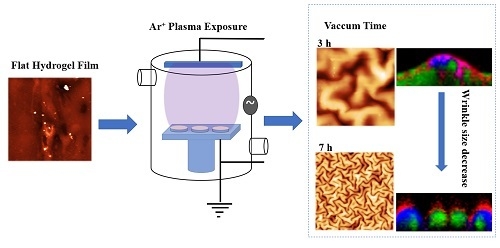
2.2. Effect of the Vacuum Exposure Time on the Wrinkle Dimensions
2.3. Plasma Exposure Time Variation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Substrate Functionalization
3.2.2. Prepolymerization of the Hydrogel (Step 1)
3.2.3. Sample Deposition (Step 2)
3.2.4. Vacuum Exposure and UV-vis Photopolymerization: Spontaneous Formation of Wrinkled Patterns (Step 3)
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, S.; Li, R.; Freire, S.G.; Garbellotto, V.M.M.; Huang, E.Y.; Smith, A.T.; Hu, C.; Tait, W.R.T.; Bian, Z.; Zheng, G.; et al. Moisture-Responsive Wrinkling Surfaces with Tunable Dynamics. Adv. Mater. 2017, 29, 1700828. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hou, H.; Yin, J.; Jiang, X. Near-infrared light–responsive dynamic wrinkle patterns. Sci. Adv. 2018, 4, eaar5762. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, J.; Yi, L. Fabrication of pH-responsive hydrophilic/hydrophobic Janus cotton fabric via plasma-induced graft polymerization. Mater. Lett. 2017, 208, 46–49. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, S.; Yang, B.; Bao, M.; Chen, G.; Zhang, X. Chemically Modified Surface Having a Dual-Structured Hierarchical Topography for Controlled Cell Growth. Acs Appl. Mater. Interfaces 2017, 9, 24339–24347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Castañeda Ocampo, O.; Guimarães, C.F.; Kühn, P.T.; Van Kooten, T.G.; Van Rijn, P. Screening Platform for Cell Contact Guidance Based on Inorganic Biomaterial Micro/nanotopographical Gradients. Acs Appl. Mater. Interfaces 2017, 9, 31433–31445. [Google Scholar] [CrossRef]
- Chen, W.; Gui, X.; Yang, L.; Zhu, H.; Tang, Z. Wrinkling of two-dimensional materials: Methods, properties and applications. Nanoscale Horiz. 2019. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J. Antimicrobial/Antifouling Surfaces Obtained by Surface Modification. In Polymers against Microorganisms; Springer International Publishing: Cham, Switzerland, 2017; pp. 95–123. [Google Scholar]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Terraza, C.A.; del Campo-García, A.; Lopez-Martinez, E.; Cortajarena, A.L.; Casado-Losada, I.; Martínez-Campos, E.; Rodríguez-Hernández, J. Design and fabrication of biocompatible wrinkled hydrogel films with selective antibiofouling properties. Mater. Sci. Eng. C 2019, 97, 803–812. [Google Scholar] [CrossRef]
- Dubey, A.K.; Yadava, V. Laser beam machining—A review. Int. J. Mach. Tools Manuf. 2008, 48, 609–628. [Google Scholar] [CrossRef]
- Queiroz, T.P.; de Molon, R.S.; Souza, F.Á.; Margonar, R.; Thomazini, A.H.A.; Guastaldi, A.C.; Hochuli-Vieira, E. In vivo evaluation of cp Ti implants with modified surfaces by laser beam with and without hydroxyapatite chemical deposition and without and with thermal treatment: Topographic characterization and histomorphometric analysis in rabbits. Clin. Oral Investig. 2017, 21, 685–699. [Google Scholar] [CrossRef]
- Lim, C.-M.; Lee, I.-K.; Lee, K.J.; Oh, Y.K.; Shin, Y.-B.; Cho, W.-J. Improved sensing characteristics of dual-gate transistor sensor using silicon nanowire arrays defined by nanoimprint lithography. Sci. Technol. Adv. Mater. 2017, 18, 17–25. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, W.; Shen, S.; Feng, L.; Deng, Y. Strain-Induced Surface Micro/Nanosphere Structure: A New Technique to Design Mechanically Robust Superhydrophobic Surfaces with Rose Petal-Like Morphology. Adv. Mater. Interfaces 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Sahin, O.; Ashokkumar, M.; Ajayan, P.M. 3 - Micro- and Nanopatterning of Biomaterial Surfaces; Elsevier Ltd.: Amsterdam, The Netherlands; ISBN 9780081022054.
- Nazarov, D.; Zemtsova, E.; Solokhin, A.; Valiev, R.; Smirnov, V. Modification of the Surface Topography and Composition of Ultrafine and Coarse Grained Titanium by Chemical Etching. Nanomaterials 2017, 7, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Glor, E.C.; Li, M.; Liu, T.; Wahid, K.; Zhang, W.; Riggleman, R.A.; Fakhraai, Z. Long-range correlated dynamics in ultra-thin molecular glass films. J. Chem. Phys. 2016, 145. [Google Scholar] [CrossRef]
- Chung, J.Y.; Douglas, J.F.; Stafford, C.M. A wrinkling-based method for investigating glassy polymer film relaxation as a function of film thickness and temperature. J. Chem. Phys. 2017, 147, 154902. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X. Beyond wrinkles: Multimodal surface instabilities for multifunctional patterning. Mrs Bull. 2016, 41, 115–122. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodríguez-Hernández, J. Wrinkled Polymer Surfaces: Strategies, Methods and Applications, 1st ed.; González-Henríquez, C.M., Rodríguez-Hernández, J., Eds.; Springer New York: New York, NY, USA, 2019; ISBN 978-3-030-05123-5. [Google Scholar]
- Rodríguez-Hernández, J. Wrinkled interfaces: Taking advantage of surface instabilities to pattern polymer surfaces. Prog. Polym. Sci. 2015, 42, 1–41. [Google Scholar] [CrossRef]
- Evensen, H.T.; Jiang, H.; Gotrik, K.W.; Denes, F.; Carpick, R.W. Transformations in Wrinkle Patterns: Cooperation between Nanoscale Cross-Linked Surface Layers and the Submicrometer Bulk in Wafer-Spun, Plasma-Treated Polydimethylsiloxane. Nano Lett. 2009, 9, 2884–2890. [Google Scholar] [CrossRef] [PubMed]
- Nania, M.; Matar, O.K.; Cabral, J.T. Frontal vitrification of PDMS using air plasma and consequences for surface wrinkling. Soft Matter 2015, 11, 3067–3075. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Vlassak, J.J.; Suo, Z. Adhesion between highly stretchable materials. Soft Matter 2016, 12, 1093–1099. [Google Scholar] [CrossRef]
- Bayley, F.A.; Liao, J.L.; Stavrinou, P.N.; Chiche, A.; Cabral, J.T. Wavefront Kinetics of Plasma Oxidation of Polydimethylsiloxane: Limits for Sub-μm Wrinkling. Soft Matter 2014, 10, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.; Yoon, S.; Moon, M.-W. Plasma-Based Nanostructuring of Polymers: A Review. Polymer (Basel) 2017, 9, 417. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Morrow, B.A.; McFarlan, A.J. Chemical reactions at silica surfaces. J. Non. Cryst. Solids 1990, 120, 61–71. [Google Scholar] [CrossRef]
- Ishida, H.; Miller, J.D. Substrate Effects on the Chemisorbed and Physisorbed Layers of Methacryl Silane Modified Particulate Minerals. Macromolecules 1984, 17, 1659–1666. [Google Scholar] [CrossRef]
- Kim, K.; Byun, Y.; Kim, C.; Kim, T.C.; Noh, D.Y.; Shin, K. Combined study of X-ray reflectivity and atomic force microscopy on a surface-grafted phospholipid monolayer on a solid. J. Colloid Interface Sci. 2005, 284, 107–113. [Google Scholar] [CrossRef]
- Kim, K.; Kim, C.; Byun, Y. Preparation of a stable phospholipid monolayer grafted onto a methacryloyl- terminated substrate as blood compatible materials. J. Biomater. Sci. Polym. Ed. 2003, 14, 887–902. [Google Scholar] [CrossRef]
- Cho, G.; Jang, J.; Moon, I.; Lee, J.; Glatzhofer, D.T. Enhanced adhesion of polypyrrole film through a novel grafting method. J. Mater. Chem. 1999, 9, 345–347. [Google Scholar] [CrossRef]
- D3359-09 Standard Test Methods for Measuring Adhesion by Tape Test. Astm 2013, 1–7. [CrossRef]
- EN ISO 2409 Paints and varnishes, cross-cut test. Br. Stand. 2007. [CrossRef]
- Nania, M.; Foglia, F.; Matar, O.K.; Cabral, J.T. Sub-100 nm wrinkling of polydimethylsiloxane by double frontal oxidation. Nanoscale 2017, 9, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Cheng, J.; Liu, Q.; Wu, X.Y.; Sun, Y. Investigation of mechanical properties of soft hydrogel microcapsules in relation to protein delivery using a MEMS force sensor. J. Biomed. Mater. Res. - Part. A 2010, 92, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.E.; Lin, K.H.; Juang, J.Y. Hertzian load-displacement relation holds for spherical indentation on soft elastic solids undergoing large deformations. Tribol. Int. 2016, 97, 71–76. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Alfaro-Cerda, P.A.; Veliz-Silva, D.F.; Sarabia-Vallejos, M.A.; Terraza, C.A.; Rodriguez-Hernandez, J. Micro-wrinkled hydrogel patterned surfaces using pH-sensitive monomers. Appl. Surf. Sci. 2018, 457, 902–913. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ayora-Cañada, M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids 2005, 134, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Tables of Spectral Data for Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 1990; ISBN 978-3-540-51202-8. [Google Scholar]
- Wasserman, S.R.; Whitesides, G.M.; Tidswell, I.M.; Ocko, B.M.; Pershan, P.S.; Axe, J.D. The Structure of Self-Assembled Monolayers of Alkylsiloxanes on Silicon: A Comparison of Results from Ellipsometry and Low-Angle X-ray Reflectivity. J. Am. Chem. Soc. 1989, 111, 5852–5861. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, E.; Shao, Y.; Xiao, Z.; Dong, Q.; Huang, J. Scalable fabrication of efficient organolead trihalide perovskite solar cells with doctor-bladed active layers. Energy Env. Sci. 2015, 8, 1544–1550. [Google Scholar] [CrossRef]
- Klapetek, P.; Valtr, M.; Nečas, D.; Salyk, O.; Dzik, P. Atomic force microscopy analysis of nanoparticles in non-ideal conditions. Nanoscale Res. Lett. 2011, 6, 514. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds used in this article are available in the laboratory of nanotechnology and advanced materials (LNnMA) located in the UTEM-Chile. |










| TSM Concentration (v/v) | Area Attached after Peeling |
|---|---|
| 0.005% | 5.3 ± 2% |
| 0.1% | 8.5 ± 3% |
| 1% | 25.0 ± 2% |
| 2% | 73.0 ± 3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Henríquez, C.M.; Veliz-Silva, D.F.; Sarabia-Vallejos, M.A.; del Campo-García, A.; Rodríguez-Hernández, J. Micrometric Wrinkled Patterns Spontaneously Formed on Hydrogel Thin Films via Argon Plasma Exposure. Molecules 2019, 24, 751. https://doi.org/10.3390/molecules24040751
González-Henríquez CM, Veliz-Silva DF, Sarabia-Vallejos MA, del Campo-García A, Rodríguez-Hernández J. Micrometric Wrinkled Patterns Spontaneously Formed on Hydrogel Thin Films via Argon Plasma Exposure. Molecules. 2019; 24(4):751. https://doi.org/10.3390/molecules24040751
Chicago/Turabian StyleGonzález-Henríquez, Carmen M., Diego F. Veliz-Silva, Mauricio A. Sarabia-Vallejos, Adolfo del Campo-García, and Juan Rodríguez-Hernández. 2019. "Micrometric Wrinkled Patterns Spontaneously Formed on Hydrogel Thin Films via Argon Plasma Exposure" Molecules 24, no. 4: 751. https://doi.org/10.3390/molecules24040751
APA StyleGonzález-Henríquez, C. M., Veliz-Silva, D. F., Sarabia-Vallejos, M. A., del Campo-García, A., & Rodríguez-Hernández, J. (2019). Micrometric Wrinkled Patterns Spontaneously Formed on Hydrogel Thin Films via Argon Plasma Exposure. Molecules, 24(4), 751. https://doi.org/10.3390/molecules24040751