The Ethanol Extract of Holotrichia diomphalia Larvae, Containing Fatty acids and Amino acids, Exerts Anti-Asthmatic Effects through Inhibition of the GATA-3/Th2 Signaling Pathway in Asthmatic Mice
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of Amino Acids, Fatty Acids, Crude Protein, and Fat in HD Ethanol Extract
2.2. Effects of HD, CsA, and Rosiglitazone on AHR Induced by Methacholine Stimulation
2.3. Histological Analysis of Lung Tissues
2.4. HD, CsA, and Rosiglitazone Attenuate Airway Eosinophil and Inflammatory Cell Infiltration into the Lung and BALF
2.5. Suppressive Effect of HD, CsA, and Rosiglitazone on the Absolute Number of Immune Cell Subsets in OVA-Induced Asthmatic Mouse Lung
2.6. Inhibitory Effect of HD, CsA, and Rosiglitazone on Th2 Cytokines in BALF and IgE Production in Serum
2.7. Suppressive Effects of HD, CsA, and Rosiglitazone on IL-5, IL-13, IL-4, Eotaxin-2, GATA-3, and Loxl2 Gene Expression in Lung Tissue
2.8. Suppressive Effects of HD, Rosiglitazone, and CsA on Intracellular ROS
2.9. Effect of HD, CsA, and Rosiglitazone on Cytokine Expression in Vitro
2.10. Effect of HD, CsA, and Rosiglitazone on the Protein Expression of Transcription Factors in EL-4 T Cells
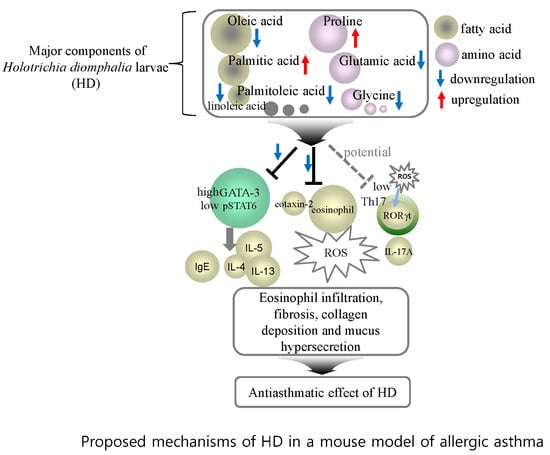
3. Discussion
4. Materials and Methods
4.1. Preparation of Ethanol Crude Extracts and Other Reagents
4.2. Amino Acids and Total Crude Protein Analysis
4.3. Fatty Acids and Total Crude Fat Analysis
4.4. Animals
4.5. OVA Sensitization, Inhalation, Challenge, and Enhanced Pause (Penh) Measurement
4.6. Bronchoalveolar Lavage Fluid (BALF)
4.7. Digestion of Lung Tissue And Cell Preparation
4.8. Hematoxylin-Eosin (H & E), Masson-Trichrome (M-T), and Periodic acid-Schiff (PAS) Staining
4.9. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Quantitative Real-Time PCR (qRT-PCR) in Vivo
4.12. Intracellular Reactive Oxygen Species (ROS) Measurement
4.13. Detection of Cytokines by ELISA and Immunoblotting Analysis in Vitro
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, N.S.; Park, S.Y.; Lee, K.R.; Lee, S.M.; Lee, B.G.; Shin, D.H.; Pyo, S. Modulation of macrophage function activity by ethanolic extract of larvae of Holotrichia diomphalia. J. Ethnopharmacol. 2002, 79, 89–94. [Google Scholar] [CrossRef]
- Zhonghua Bencao Editorial Committee. State Administration of Traditional Chinese Medicine (Zhonghua). Zhonghua Bencao (The Chinese Herbal); Shanghai Scientific and Technical Press: Shanghai, China, 1999; pp. 129–231. [Google Scholar]
- Lee, S.Y.; Moon, H.J.; Kurata, S.; Kurama, T.; Natori, S.; Lee, B.L. Purification and molecular cloning of cDNA for an inducible antibacterial protein of larvae of a coleopteran insect, Holotrichia diomphalia. J. Biochem. 1994, 115, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Pyo, S.; Lee, K.R.; Lee, B.K.; Shin, D.H.; Cho, S.I.; Lee, S.M. Effect of Holotrichia diomphalia larvae on liver fibrosis and hepatotoxicity in rats. J. Ethnopharmacol. 2003, 87, 175–180. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Li, W.; Liu, S. Anticoagulant activity of crude extract of Holotrichia diomphalia larvae. J. Ethnopharmacol. 2016, 177, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, D.; Zhang, Y.K.; Wang, X.Y.; Chang, Y.R.; Yang, Q.; Wang, S.W. Analysis of proteins, amino acids and inorganic elements in Holotrichia diomphalia from different areas. Zhong Yao Cai 2010, 33, 1538–1541. [Google Scholar] [PubMed]
- Pei, K.; Cao, W.; Guo, Q.Q.; Xie, Y.H.; He, Z.M.; Wang, S.W. Analysis of liposoluble constituents in Holotrichia diomphalia by GC-MS and investigation their anti-inflammatory and analgesic activities. Zhong Yao Cai 2012, 35, 357–360. [Google Scholar] [PubMed]
- Kompauer, I.; Demmelmair, H.; Koletzko, B.; Bolte, G.; Linseisen, J.; Heinrich, J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur. J. Epidemiol. 2008, 23, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Ledón, N.; Romay, C.H.; Rodríguez, V.; Cruz, J.; Rodríguez, S.; Ancheta, O.; González, A.; González, R.; Tolón, Z.; Cano, M.; et al. Further studies on a mixture of fatty acids from sugar cane (Saccharum officinarum) wax oil in animal models of hypersensitivity. Planta Med. 2005, 71, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just T(H)2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, A.D.; Diamant, Z.; Hanania, N.A. Role of T2 inflammation biomarkers in severe asthma. Curr. Opin. Pulm. Med. 2016, 22, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Lawrence, M.G. T-cell biology in immunotherapy. Ann. Allergy Asthma Immunol. 2014, 112, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deckers, J.; Branco Madeira, F.; Hammad, H. Innate immune cells in asthma. Trends Immunol. 2013, 34, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Fish, S.C.; Donaldson, D.D.; Goldman, S.J.; Williams, C.M.; Kasaian, M.T. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J. Immunol. 2005, 174, 7716–7724. [Google Scholar] [CrossRef] [PubMed]
- Saatian, B.; Rezaee, F.; Desando, S.; Emo, J.; Chapman, T.; Knowlden, S.; Georas, S.N. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers 2013, 1, e24333. [Google Scholar] [CrossRef] [PubMed]
- Chakir, H.; Wang, H.; Lefebvre, D.E.; Webb, J.; Scott, F.W. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: Predominant role of GATA-3. J. Immunol. Methods 2003, 278, 157–169. [Google Scholar] [CrossRef]
- Zheng, W.; Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Ting, C.N.; Olson, M.C.; Barton, K.P.; Leiden, J.M. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature 1996, 384, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Asakawa, J.; Motojima, S.; Makino, S. Cyclosporine A reduces T lymphocyte activity and improves airway hyperresponsiveness in corticosteroid-dependent chronic severe asthma. Ann. Allergy Asthma Immunol. 1995, 75, 65–72. [Google Scholar] [PubMed]
- Alexander, A.G.; Barnes, N.C.; Kay, A.B. Trial of cyclosporin in corticosteroid-dependent chronic severe asthma. Lancet 1992, 339, 324–328. [Google Scholar] [CrossRef]
- Woerly, G.; Honda, K.; Loyens, M.; Papin, J.P.; Auwerx, J.; Staels, B.; Capron, M.; Dombrowicz, D. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J. Exp. Med. 2003, 198, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, Y.A.; Hanson, K.M.; Keles, S.; Brockman-Schneider, R.A.; Jarjour, N.N.; Gern, J.E. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010, 3, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Yang, C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Caramori, G.; Chung, K.F. New targets for drug development in asthma. Lancet 2008, 372, 1073–1087. [Google Scholar] [CrossRef]
- Eynott, P.R.; Salmon, M.; Huang, T.J.; Oates, T.; Nicklin, P.L.; Chung, K.F. Effects of cyclosporin A and a rapamycin derivative (SAR943) on chronic allergic inflammation in sensitized rats. Immunology 2003, 109, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.L.; Ju, J.H.; Kim, K.W.; Moon, Y.M.; Lee, S.Y.; Min, S.Y.; Cho, Y.G.; Kim, H.S.; Park, K.S.; Yoon, C.H.; et al. Cyclosporine A inhibits IL-15-induced IL-17 production in CD4+ T cells via downregulation of PI3K/Akt and NF-kappaB. Immunol. Lett. 2007, 108, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Baker, K.; Dale, N.; Dubuis, E.; Shala, F.; Belvisi, M.G.; Birrell, M.A. CD4+ and CD8+ T cells play a central role in a HDM driven model of allergic asthma. Respir. Res. 2016, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Kemp, R.A.; Ronchese, F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J. Immunol. 2001, 167, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, N.; Swanson, B.J.; Takeda, K.; Taube, C.; Miyahara, S.; Kodama, T.; Dakhama, A.; Ott, V.L.; Gelfand, E.W. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat. Med. 2004, 10, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Gurary, A.; Hoffmann, F.W.; Jourdan-Le Saux, C.; Teeters, K.; Hashimoto, A.C.; Tam, E.K.; Berry, M.J. A new approach for analyzing cellular infiltration during allergic airway inflammation. J. Immunol. Methods 2007, 328, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki-Hosokawa, T.; Hasegawa, A.; Iwamura, C.; Shinoda, K.; Tofukuji, S.; Watanabe, Y.; Hosokawa, H.; Motohashi, S.; Hashimoto, K.; Shirai, M.; et al. CD69 controls the pathogenesis of allergic airway inflammation. J. Immunol. 2009, 183, 8203–8215. [Google Scholar] [CrossRef] [PubMed]
- Vale-Pereira, S.; Todo-Bom, A.; Geraldes, L.; Schmidt-Weber, C.; Akdis, C.A.; Mota-Pinto, A. FoxP3, GATA-3 and T-bet expression in elderly asthma. Clin. Exp. Allergy 2011, 41, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Chesné, J.; Braza, F.; Mahay, G.; Brouard, S.; Aronica, M.; Magnan, A. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 2014, 190, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, S.; Lewkowich, I.P.; Suzuki, Y.; Clark, J.R.; Sproles, A.A.; Dienger, K.; Budelsky, A.L.; Wills-Karp, M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 2010, 11, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Thai, P.; Zhao, Y.H.; Ho, Y.S.; DeSouza, M.M.; Wu, R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003, 278, 17036–17043. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 1996, 4, 313–319. [Google Scholar] [CrossRef]
- Zimmermann, N.; Hogan, S.P.; Mishra, A.; Brandt, E.B.; Bodette, T.R.; Pope, S.M.; Finkelman, F.D.; Rothenberg, M.E. Murine eotaxin-2: A constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J. Immunol. 2000, 165, 5839–5846. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, A.; Kanehiro, A.; Rabinovitch, N.; Joetham, A.; Cieslewicz, G.; Gelfand, E.W. The failure of STAT6 deficient mice to develop airway eosinophilia and airway hyperresponsiveness is overcome by interleukin-5. Am. J. Respir. Crit. Care Med. 1999, 160, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Duschl, A.; Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 2012, 42, 2827–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayees, S.; Malik, F.; Bukhari, S.I.; Singh, G. Linking GATA-3 and interleukin-13: Implications in asthma. Inflamm. Res. 2014, 63, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Meng, Q.; Zeibecoglou, K.; Robinson, D.S.; Macfarlane, A.; Humbert, M.; Kay, A.B. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J. Immunol. 1999, 163, 6321–6329. [Google Scholar] [PubMed]
- McGuffin, M. Should herbal medicines be regulated as drugs? Clin. Pharmacol. Ther. 2008, 83, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Lumia, M.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Erkkola, M.; Uusitalo, L.; Niinistö, S.; Kenward, M.G.; Ilonen, J.; Simell, O.; et al. Dietary fatty acid composition during pregnancy and the risk of asthmain the offspring. Pediatr. Allergy Immunol. 2011, 22, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Lekogo, B.M.; Coroller, L.; Mathot, A.G.; Mafart, P.; Leguerinel, I. Modelling the influence of palmitic, palmitoleic, stearic and oleic acids on apparent heat resistance of spores of Bacillus cereus NTCC 11145 and Colostridium sporogenes Pasteur 79.3. Int. J. Food Microbiol. 2010, 141, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.M.; Rodríguez-Landa, J.F.; Gutiérrez-García, A.G.; Mendoza-López, M.R.; García-Ríos, R.I.; Cueto-Escobedo, J. Anxiolytic-like effects of human amniotic fluid and its fatty acids in Wistar rats. Behav. Pharmacol. 2011, 22, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Mattison, C.P.; Reed, S.; Wasserman, R.L.; Desormeaux, W.A. Treatment with oleic acid reduces IgE binding to peanut and cashewallergens. Food Chem. 2015, 180, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, A.M.; Ylönen, N.; von Willebrand, E.; Basu, S.; Aro, A. Immunological and metabolic effects of cis-9, trans-11-conjugated linoleic acid in subjects with birch pollen allergy. Br. J. Nutr. 2008, 100, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Jaudszus, A.; Krokowski, M.; Möckel, P.; Darcan, Y.; Avagyan, A.; Matricardi, P.; Jahreis, G.; Hamelmann, E. Cis-9,trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a PPARgamma-related mechanism in mice. J. Nutr. 2008, 138, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Bae, C.S.; Seo, N.S.; Na, C.S.; Yoo, H.Y.; Oh, D.S.; Bae, M.S.; Kwon, M.S.; Cho, S.S.; Park, D.H. Camellia japonica oil suppressed asthma occurrence via GATA-3 & IL-4 pathway and its effective and major component is oleic acid. Phytomedicine 2018, 57, 84–94. [Google Scholar] [PubMed]
- Katagiri, K.; Arakawa, S.; Kurahashi, R. IL-4 restores impaired contact hypersensitivity response in obese mice fed a high-fat diet enriched with oleic acid. J. Invest. Dermatol. 2008, 128, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Warren, J.M.; Simon, V.A.; Bartolini, G.; Mackey, B.E.; Erickson, K.L. Similar effects of c9,t11-CLA and t10,c12-CLA on immune cell functions in mice. Lipids 2002, 37, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Tachdjian, R.; Al Khatib, S.; Schwinglshackl, A.; Kim, H.S.; Chen, A.; Blasioli, J.; Mathias, C.; Kim, H.Y.; Umetsu, D.T.; Oettgen, H.C.; et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J. Allergy Clin. Immunol. 2010, 125, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, S.H.; Yoon, H.J.; Paik, D.J.; Kim, J.M.; Youn, J. Bacillus-derived poly-γ-glutamic acid attenuates allergic airway inflammation through a Toll-like receptor-4-dependent pathway in a murine model of asthma. Clin. Exp. Allergy 2011, 41, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Trak-Fellermeier, M.A.; Brasche, S.; Winkler, G.; Koletzko, B.; Heinrich, J. Food and fatty acid intake and atopic disease in adults. Eur. Respir. J. 2004, 23, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, C.O.; Teixeira, A.A.; Biondo, L.A.; Silveira, L.S.; Calder, P.C.; Rosa-Neto, J.C. Palmitoleic acid reduces the inflammation in LPS-stimulated macrophages by inhibition of NFκB, independently of PPARs. Clin. Exp. Pharmacol. Physiol. 2017, 44, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Takahashi, K.; Sadamatsu, H.; Kato, G.; Kurata, K.; Kimura, S.; Sueoka-Aragane, N. Saturated Fatty acid increases lung macrophages and augments house dust mite-induced Airway Inflammation in Mice Fed with High-Fat Diet. Inflammation 2017, 40, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Daniliak, I.G.; Kogan, A.K.; Bolevich, S. Aevit and glutamic acid in the treatment of patients with bronchial asthma. Klin. Med. 1995, 73, 50–53. [Google Scholar]
- Hanazawa, T.; Kharitonov, S.A.; Barnes, P.J. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.C.; Hardy, K.A.; Morris, C.R. Arginase and arginine dysregulation in asthma. J. Allergy 2011, 2011, 736319. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Hwang, Y.H.; Lee, H.S.; Kim, Y.; Shin, T.H.; Lee, G.; Son, Y.J.; Kim, H.; Yee, S.T.; Park, A.K.; et al. Metabolomic study for monitoring of biomarkers in mouse plasma with asthma by gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1063, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Otenbaker, N.P.; Rose, B.A.; Salisbury, K.S. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol. Immunol. 2013, 56, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Al-Harbi, M.M.; Ansari, M.A.; AlSharari, S.D.; Bahashwan, S.A.; Attia, S.M.; Al-Hosaini, K.A.; Al Hoshani, A.R.; Ahmad, S.F. Airway oxidative stress causes vascular and hepatic inflammation via upregulation of IL-17A in a murine model of allergic asthma. Int. Immunopharmacol. 2016, 34, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Yang, Q. Antioxidant activity and phenolic compounds of Holotrichia parallela Motschulsky extracts. Food Chem. 2012, 134, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gonzalez, I.; Miranda-Becerra, D.; Montano-Benavides, S. Evaluation of phosphorus, protein, and n-3 fatty acid content in 15 marine fish species identifies the species most beneficial to renal patients. J. Ren. Nutr. 2009, 19, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Vireque, A.A.; Ferreira, C.R.; Hatanaka, R.R.; Tata, A.; Belaz, K.R.A.; Santos, V.G.; Eberlin, M.N.; Silva-de-Sá, M.F.; Ferriani, R.A.; Rosa-E-Silva, A.C. Dataset on lipid profile of bovine oocytes exposed to Lα-phosphatidylcholine during in vitro maturation investigated by MALDI mass spectrometry and gas chromatography-flame ionization detection. Data Brief 2017, 13, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, J.H.; Lee, J.E.; Lee, Y.C. 18β-Glycyrrhetinic acid, the major bioactive component of Glycyrrhizae Radix, attenuates airway inflammation by modulating Th2 cytokines, GATA-3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environ. Toxicol. Pharmacol. 2017, 52, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, J.H.; Lee, Y.C. Oleanolic acid suppresses ovalbumin induced airway inflammation and Th2-mediated allergic asthma by modulating the transcription factors T-bet, GATA-3, RORγt and Foxp3 in asthmatic mice. Int. Immunopharmacol. 2014, 18, 311–324. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








| Compounds (Amino Acid) | Formula | Result (mg/100g) |
|---|---|---|
| Aspartic acid | C4H7NO4 | 962.9 |
| Threonine | C4H9NO3 | 806.4 |
| Serine | C3H7NO3 | 1731.3 |
| Glutamic acid | C5H9NO4 | 3210.3 |
| Proline | C5H9NO2 | 5375.1 |
| Glycine | C2H5NO2 | 1771.1 |
| Alanine | C3H7NO2 | 1675.5 |
| Valine | C5H11NO2 | 1241.5 |
| Methionine | C5H11NO2S | 300.9 |
| Isoleucine | C6H13NO2 | 739.2 |
| Leucine | C6H13NO2 | 806.5 |
| Tyrosine | C9H11NO3 | 4009.2 |
| Phenylalanine | C9H11NO2 | 873.8 |
| Lysine | C6H14N2O2 | 855.4 |
| Histidine | C6H9N3O2 | 579.9 |
| Arginine | C6H14N4O2 | 786.6 |
| Total crude protein (%) | 29.95 |
| Compounds (Fatty Acid) | Formula | Result (g/100g) |
|---|---|---|
| Lauric acid | C12H24O2 | 0.005 |
| Tridecanoic acid | C13H26O2 | 0.003 |
| Myristic acid | C14H28O2 | 0.180 |
| Myristoleic acid | C14H26O2 | 0.026 |
| Pentadecanoic acid | C15H30O2 | 0.046 |
| Pentadecenoic acid | C15H28O2 | 0.001 |
| Palmitic acid | C16H32O2 | 3.206 |
| Palmitoleic acid | C16H30O2 | 1.792 |
| Margaric acid | C17H34O2 | 0.069 |
| Heptadecenoic acid | C17H34O2 | ND |
| Stearic acid | C18H36O2 | 0.349 |
| Oleic acid | C18H34O2 | 0.046 (trans) |
| 8.893 (cis) | ||
| Linoleic acid | C18H32O2 | 0.059 (trans) |
| 1.076 (cis) | ||
| Arachidic acid | C20H40O2 | 0.078 |
| γ-Linolenic acid | C20H34O2 | ND |
| Linolenic acid | C18H30O2 | 0.172 |
| Gadoleic acid | C20H38O2 | 0.026 |
| Heneicosanoic acid | C21H42O2 | ND |
| Eicosadienoic acid | C20H36O2 | 0.009 |
| Behenic acid | C21H43COOH | 0.013 |
| Dihomo-gamma-linolenic acid | C20H34O2 | 0.010 |
| Erucic acid | C22H42O2 | 0.006 |
| Eicosatrienoic acid | C20H34O2 | ND |
| Arachidoninc acid | C20H32O2 | 0.171 |
| Tricosanoic acid | C23H46O2 | ND |
| Brassic acid | C22H40O2 | ND |
| Lignoceric acid | C24H48O2 | 0.004 |
| EPA | C20H30O2 | 0.060 |
| Nervonic acid | C24H46O2 | ND |
| DHA | C22H32O2 | 0.005 |
| Total crude fat (%) | 15.92 |
| Cell Phenotypes in Lung | Normal BALB/c | Ovalbumin-Induced Asthma Mice (Absolute No.) | |||||
|---|---|---|---|---|---|---|---|
| Control | Cyclosporin A | Rosi. (2 mg/kg) | HD (100 mg/kg) | HD (300 mg/kg) | |||
| CD3+/CD4+ (×105 cells) | 184.30 ± 83.61 | 633.00 ± 10.17 | 351.40 ± 85.95 ** | 374.60± 12.82 *** | 288.60± 65.99 ** | 350.60 ± 46.97 ** | |
| CD3+/CD8+ (×105 cells) | 54.60± 30.83 | 201.90 ± 1.09 | 140.70 ± 22.55 * | 123.20 ± 11.27 *** | 117.40 ± 29.85 * | 133.70 ± 33.87 * | |
| Gr-1+/CD11b+ (×104 cells) | Lung | 72.80 ± 28.69 | 228.00 ± 3.14 | 150.40 ± 37.44 * | 337.70 ± 49.72 | 124.10 ± 28.21 ** | 185.10 ± 5.18 *** |
| CD4+/CD69+ (×105 cells) | 1.70 ± 1.39 | 20.30 ± 0.80 | 39.50 ± 15.98 | 12.10± 0.72 *** | 10.60± 3.77 ** | 12.10 ± 2.63 ** | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-H.; Kim, S.-H.; Lee, Y.-C. The Ethanol Extract of Holotrichia diomphalia Larvae, Containing Fatty acids and Amino acids, Exerts Anti-Asthmatic Effects through Inhibition of the GATA-3/Th2 Signaling Pathway in Asthmatic Mice. Molecules 2019, 24, 852. https://doi.org/10.3390/molecules24050852
Hong J-H, Kim S-H, Lee Y-C. The Ethanol Extract of Holotrichia diomphalia Larvae, Containing Fatty acids and Amino acids, Exerts Anti-Asthmatic Effects through Inhibition of the GATA-3/Th2 Signaling Pathway in Asthmatic Mice. Molecules. 2019; 24(5):852. https://doi.org/10.3390/molecules24050852
Chicago/Turabian StyleHong, Jung-Hee, Seung-Hyung Kim, and Young-Cheol Lee. 2019. "The Ethanol Extract of Holotrichia diomphalia Larvae, Containing Fatty acids and Amino acids, Exerts Anti-Asthmatic Effects through Inhibition of the GATA-3/Th2 Signaling Pathway in Asthmatic Mice" Molecules 24, no. 5: 852. https://doi.org/10.3390/molecules24050852
APA StyleHong, J. -H., Kim, S. -H., & Lee, Y. -C. (2019). The Ethanol Extract of Holotrichia diomphalia Larvae, Containing Fatty acids and Amino acids, Exerts Anti-Asthmatic Effects through Inhibition of the GATA-3/Th2 Signaling Pathway in Asthmatic Mice. Molecules, 24(5), 852. https://doi.org/10.3390/molecules24050852