One-Pot NBS-Promoted Synthesis of Imidazoles and Thiazoles from Ethylarenes in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions for Synthesis of Imi-azo[1,2-a]pyridine 3a
2.2. Substrate Scope for the Imidazo[1,2-a]pyridines
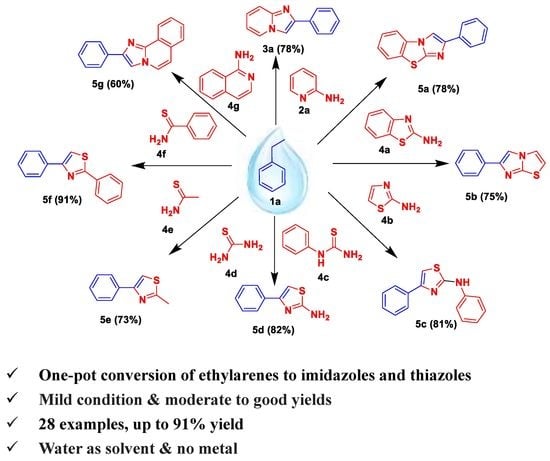
2.3. Substrate Scope for Diverse Imidazoles and Thiazoles
2.4. Gram-Scale Preparation and Practice Application
2.5. Mechanism
3. Materials and Methods
3.1. General Information
3.2. Experimental Part Method
3.2.1. General Procedure for the Synthesis of Nitrogen-Containing Heterocycles
3.2.2. Procedure for the Synthesis of 1-Ethyl-4-(methylsulfonyl)benzene (1h)
3.3. Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Katritzky, A.R.; Xu, Y.J.; Tu, H. Regiospecific synthesis of 3-substituted imidazo[1,2-a]pyridines, imidazo[1,2-a]pyrimidines, and imidazo[1,2-c]pyrimidine. J. Org. Chem. 2003, 68, 4935–4937. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Rodriguez Rivero, M.; Buchwald, S.L. Domino cu-catalyzed C--N coupling/hydroamidation: A highly efficient synthesis of nitrogen heterocycles. Angew. Chem. Int. Ed. 2006, 45, 7079–7082. [Google Scholar] [CrossRef] [PubMed]
- Naito, T. Development of new synthetic reactions for nitrogen-containing compounds and their application. Chem. Pharm. Bull. 2008, 56, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Almirante, L.; Polo, L.; Mugnaini, A.; Provinciali, E.; Rugarli, P.; Biancotti, A.; Gamba, A.; Murmann, W. Derivatives of Imidazole. I. Synthesis and Reactions of Imidazo(1,2-a)Pyridines with Analgesic, Anti-Inflammatory, Antipyretic, and Anticonvulsant Activity. J. Med. Chem. 1965, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kanakasabai, S.; Bright, J.J. Constitutive androstane receptor agonist CITCO inhibits growth and expansion of brain tumour stem cells. Br. J. Cancer 2011, 104, 448–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, S.Z.; Arbilla, S.; Benavides, J.; Scatton, B. Zolpidem and alpidem: Two imidazopyridines with selectivity for omega 1- and omega 3-receptor subtypes. Adv. Biochem. Psychopharmacol. 1990, 46, 61–72. [Google Scholar] [PubMed]
- Maruyama, Y.; Anami, K.; Terasawa, M.; Goto, K.; Imayoshi, T.; Kadobe, Y.; Mizushima, Y. Anti-inflammatory activity of an imidazopyridine derivative (miroprofen). Arzneimittelforschung 1981, 31, 1111–1118. [Google Scholar] [PubMed]
- Tomioka, K.; Yamada, T.; Mase, T.; Murase, K. Pharmacological properties of YM-11124, a selective immunosuppressive agent for cell-mediated immunity. Pharmacology 1989, 39, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Gunaganti, N.; Kharbanda, A.; Lakkaniga, N.R.; Zhang, L.; Cooper, R.; Li, H.Y.; Frett, B. Catalyst free, C-3 functionalization of imidazo[1,2-a]pyridines to rapidly access new chemical space for drug discovery efforts. Chem. Commun. 2018, 54, 12954–12957. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Liang, J.J.; Yang, S.; Chen, H.; Fu, Y.N.; Han, S.L.; Zhao, Y.H. Palladium-catalyzed oxidative C-H/C-H cross-coupling of imidazopyridines with azoles. Org. Biomol. Chem. 2018, 16, 6039–6046. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Lu, S.; Tian, L.L.; Huang, E.L.; Hao, X.Q.; Zhu, X.; Shao, T.; Song, M.P. Iodine-Mediated Difunctionalization of Imidazopyridines with Sodium Sulfinates: Synthesis of Sulfones and Sulfides. J. Org. Chem. 2018, 83, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Kitson, P.J.; Marie, G.; Francoia, J.P.; Zalesskiy, S.S.; Sigerson, R.C.; Mathieson, J.S.; Cronin, L. Digitization of multistep organic synthesis in reactionware for on-demand pharmaceuticals. Science 2018, 359, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Lefin, R.; van der Walt, M.M.; Milne, P.J.; Terre’Blanche, G. Imidazo[1,2-alpha]pyridines possess adenosine A1 receptor affinity for the potential treatment of cognition in neurological disorders. Bioorg. Med. Chem. Lett. 2017, 27, 3963–3967. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.M.; Peese, K.M. General Method for the Preparation of Electron-Deficient Imidazo[1,2-a]pyridines and Related Heterocycles. Org. Lett. 2015, 17, 6002–6005. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Mu, S.; Liu, Z.; Feng, R.; Li, Y.; Pang, K.; Zhang, B. Copper-catalyzed C-N bond formation with imidazo[1,2-a]pyridines. Org. Biomol. Chem. 2018, 16, 6655–6658. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Kayakiri, H.; Satoh, S.; Inoue, T.; Sawada, Y.; Imai, K.; Inamura, N.; Asano, M.; Hatori, C.; Katayama, A.; et al. A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 1. Construction of the basic framework. J. Med. Chem. 1998, 41, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Park, B.K.; Lim, H.; Kim, H.P.; Chang, H.W.; Jeong, J.H.; Park, H. Synthesis of biflavones having a 6-O-7’’ linkage and effects on cyclooxygenase-2 and inducible nitric oxide synthase. Bioorg. Med. Chem. Lett. 2009, 19, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Fookes, C.J.; Pham, T.Q.; Mattner, F.; Greguric, I.; Loc’h, C.; Liu, X.; Berghofer, P.; Shepherd, R.; Gregoire, M.C.; Katsifis, A. Synthesis and biological evaluation of substituted [18F]imidazo[1,2-a]pyridines and [18F]pyrazolo[1,5-a]pyrimidines for the study of the peripheral benzodiazepine receptor using positron emission tomography. J. Med. Chem. 2008, 51, 3700–3712. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.C.; Gancia, E.; Gilligan, M.T.; Goodacre, S.; Hallett, D.; Merchant, K.J.; Thomas, S.R. 8-Fluoroimidazo[1,2-a]pyridine: Synthesis, physicochemical properties and evaluation as a bioisosteric replacement for imidazo[1,2-a]pyrimidine in an allosteric modulator ligand of the GABA A receptor. Bioorg. Med. Chem. Lett. 2006, 16, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Rupert, K.C.; Henry, J.R.; Dodd, J.H.; Wadsworth, S.A.; Cavender, D.E.; Olini, G.C.; Fahmy, B.; Siekierka, J.J. Imidazopyrimidines, potent inhibitors of p38 MAP kinase. Bioorg. Med. Chem. Lett. 2003, 13, 347–350. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Santra, S.; Monir, K.; Hajra, A. Synthesis of imidazo[1,2-a]pyridines: A decade update. Chem. Commun. 2015, 51, 1555–1575. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, N.; Gevorgyan, V. General and efficient copper-catalyzed three-component coupling reaction towards imidazoheterocycles: One-pot synthesis of alpidem and zolpidem. Angew. Chem. Int. Ed. 2010, 49, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, N.K.; Pericherla, K.; Kaswan, P.; Kumar, A. Synthesis of novel azole-fused quinazolines via one-pot, sequential Ullmann-type coupling and intramolecular dehydrogenative C-N bonding. Org. Biomol. Chem. 2015, 13, 2947–5290. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.L.; Yan, H.; Ma, C.; Ren, Z.Y.; Gao, X.A.; Huang, G.S.; Liang, Y.M. Cu(I)-catalyzed synthesis of imidazo[1,2-a]pyridines from aminopyridines and nitroolefins using air as the oxidant. J. Org. Chem. 2012, 77, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Wu, W.; Zhang, Y.; Su, W. CuI-catalyzed aerobic oxidative alpha-aminaton cyclization of ketones to access aryl or alkenyl-substituted imidazoheterocycles. J. Org. Chem. 2013, 78, 12494–12504. [Google Scholar] [CrossRef] [PubMed]
- Talbot, E.P.; Richardson, M.; McKenna, J.M.; Toste, F.D. Gold Catalysed Redox Synthesis of Imidazo[1,2-a]pyridine using Pyridine N-Oxide and Alkynes. Adv. Synth. Catal. 2014, 356, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Alajarin, M.; Cabrera, J.; Pastor, A.; Sanchez-Andrada, P.; Bautista, D. On the [2+2] cycloaddition of 2-aminothiazoles and dimethyl acetylenedicarboxylate. Experimental and computational evidence of a thermal disrotatory ring opening of fused cyclobutenes. J. Org. Chem. 2006, 71, 5328–5339. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.H.; Lusi, A. Imidazo(1,2-a)pyridine anthelmintic and antifungal agents. J. Med. Chem. 1972, 15, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Shinde, M.H.; Kshirsagar, U.A. One pot synthesis of substituted imidazopyridines and thiazoles from styrenes in water assisted by NBS. Green Chem. 2016, 18, 1455–1458. [Google Scholar] [CrossRef]
- Shimokawa, S.; Kawagoe, Y.; Moriyama, K.; Togo, H. Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I2-Aqueous NH3. Org. Lett. 2016, 18, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: A decade update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jun, J.-G. An Efficient Copper-catalyzed Nucleophilic Addition to N-Acyliminium Ions Derived from N-Benzyloxycarbonylamino Sulfones: A Novel Approach to C-3 Functionalization of 2-Phenylimidazo[1,2-a]pyridine. Bulletin Korean Chem. Soc. 2017, 38, 1123–1128. [Google Scholar] [CrossRef]
- Redon, S.; Obah Kosso, A.R.; Broggi, J.; Vanelle, P. Easy and efficient selenocyanation of imidazoheterocycles using triselenodicyanide. Tetrahedron Lett. 2017, 58, 2771–2773. [Google Scholar] [CrossRef] [Green Version]
- Parisio, C.; Clementi, F. Surface alterations induced by stress in gastric mucosa: Protective effect of zolimidine. A transmission and scanning electron microscope investigation. Lab. Investig. 1976, 35, 484–495. [Google Scholar] [PubMed]
- Belohlavek, D.; Malfertheiner, P. The effect of zolimidine, imidazopyridine-derivate, on the duodenal ulcer healing. Scand. J. Gastroenterol Suppl. 1979, 54, 44. [Google Scholar] [PubMed]
- Materia, A.; Basso, N.; Bagarani, M.; Basoli, A.; Speranza, V. Treatment of alkaline reflux gastritis with zolimidine. Controlled study. Clin. Ter. 1981, 97, 183–191. [Google Scholar] [PubMed]
- Yuan, G.; Zheng, J.; Gao, X.; Li, X.; Huang, L.; Chen, H.; Jiang, H. Copper-catalyzed aerobic oxidation and cleavage/formation of C-S bond: A novel synthesis of aryl methyl sulfones from aryl halides and DMSO. Chem. Commun. 2012, 48, 7513–7515. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.B.; Telvekar, V.N. NBS mediated protocol for the synthesis of N -bridged fused heterocycles in water. Tetrahedron Lett. 2017, 58, 3662–3666. [Google Scholar] [CrossRef]
- Djerassi, C. Brominations with N-bromosuccinimide and related compounds; the Wohl-Ziegler reaction. Chem. Rev. 1948, 43, 271–317. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-de-Castro, A.; Xiao, J. Green and Efficient: Iron-Catalyzed Selective Oxidation of Olefins to Carbonyls with O2. J. Am. Chem. Soc. 2015, 137, 8206–8218. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |






 | ||||||
|---|---|---|---|---|---|---|
| Entry | First Step | Second Step | ||||
| Oxidant | Solvent | Solvent | Temp. (°C) | Base (Equiv.) | Yield b (%) | |
| 1 | NBS | EA/H2O (5:1) | EA/H2O (5:1) | 80 | none | 38 |
| 2 | NCS | EA/H2O (5:1) | EA/H2O (5:1) | 80 | none | 0 |
| 3 | NIS | EA/H2O (5:1) | EA/H2O (5:1) | 80 | none | 0 |
| 4 | BrNPhth | EA/H2O (2:1) | EA/H2O (5:1) | 80 | none | 34 |
| 5 | NBS | MeCN/H2O (5:1) | MeCN/H2O (5:1) | 75 | none | 33 |
| 6 | NBS | Acetone/H2O (5:1) | Acetone/H2O (5:1) | 50 | none | 28 |
| 7 | NBS | 1,4-Dioxane/H2O (5:1) | 1,4-Dioxane/H2O (5:1) | 95 | none | trace |
| 8 | NBS | EA/H2O (5:1) | H2O | 80 | none | 67 |
| 9 | NBS | EA/H2O (5:1) | EtOH | 70 | none | 65 |
| 10 | NBS | EA/H2O (5:1) | Acetone | 50 | none | 62 |
| 11 | NBS | EA/H2O (5:1) | H2O | 80 | Na2CO3 (2) | 72 |
| 12 | NBS | EA/H2O (5:1) | H2O | 80 | Na2CO3 (5) | 78 |
| 13 | NBS | EA/H2O (5:1) | H2O | reflux | Na2CO3 (5) | 77 |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhu, H.; Wang, J.; Liu, H. One-Pot NBS-Promoted Synthesis of Imidazoles and Thiazoles from Ethylarenes in Water. Molecules 2019, 24, 893. https://doi.org/10.3390/molecules24050893
Chen L, Zhu H, Wang J, Liu H. One-Pot NBS-Promoted Synthesis of Imidazoles and Thiazoles from Ethylarenes in Water. Molecules. 2019; 24(5):893. https://doi.org/10.3390/molecules24050893
Chicago/Turabian StyleChen, Liang, Huajian Zhu, Jiang Wang, and Hong Liu. 2019. "One-Pot NBS-Promoted Synthesis of Imidazoles and Thiazoles from Ethylarenes in Water" Molecules 24, no. 5: 893. https://doi.org/10.3390/molecules24050893
APA StyleChen, L., Zhu, H., Wang, J., & Liu, H. (2019). One-Pot NBS-Promoted Synthesis of Imidazoles and Thiazoles from Ethylarenes in Water. Molecules, 24(5), 893. https://doi.org/10.3390/molecules24050893