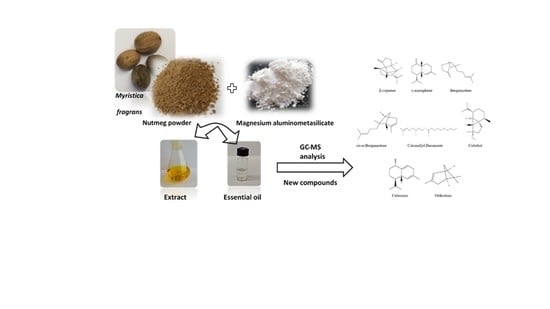
GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Maceration
4.3. Ultrasound Assisted Extraction
4.4. Hydrodistillation
4.5. Gas Chromatography-Mass Spectrometry Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tajuddin, S.; Ahmad, A.; Latif, I.; Qasmi, A.; Amin, K.M.Y. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement. Altern. Med. 2005, 5, 16–23. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Ultrasound-assisted micellar extraction of phenolic antioxidants from spices and antioxidant properties of the extracts based on coulometric titration data. Anal. Methods 2016, 8, 7150–7157. [Google Scholar] [CrossRef] [Green Version]
- Adiani, V.; Gupta, S.; Chatterjee, S.; Variyar, P.S.; Sharma, A. Activity guided characterization of antioxidant components from essential oil of Nutmeg (Myristica fragrans). J. Food Sci. Technol. 2015, 52, 221–230. [Google Scholar] [CrossRef]
- D’Souza, S.P.; Chavannavar, S.V.; Kanchanashri, B.; Niveditha, S.B. Pharmaceutical Perspectives of Spices and Condiments as Alternative Antimicrobial Remedy. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Nagja, T.; Vimal, K.; Sanjeev, A. Myristica Fragrans: A Comprehensive Review. Int. J. Pharm. Pharm. Sci. 2016, 8, 9–12. [Google Scholar]
- Dupuy, N.; Molinet, J.; Mehl, F.; Nanlohy, F.; Le Dréau, Y.; Kister, J. Chemometric analysis of mid infrared and gas chromatography data of Indonesian nutmeg essential oils. Ind. Crop. Prod. 2013, 43, 596–601. [Google Scholar] [CrossRef]
- Morsy, N.F.S. A comparative study of nutmeg (Myristica fragrans Houtt.) oleoresins obtained by conventional and green extraction techniques. J. Food Sci. Technol. 2016, 53, 3770–3777. [Google Scholar] [CrossRef] [Green Version]
- Piaru, S.P.; Mahmud, R.; Majid, A.M.S.; Nassar, Z.D. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Trop. Med. 2012, 5, 294–298. [Google Scholar]
- Djilani, A.; Dicko, A. The Therapeutic Benefits of Essential Oils. In Nutrition, Well-Being and Health; Bouayed, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 155–178. [Google Scholar]
- Barceloux, D.G. Nutmeg (Myristica fragrans Houtt.). Dis. Mon. 2009, 55, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lanari, D.; Marcotullio, M.; Neri, A. A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits. Molecules 2018, 23, 2817. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C. R. Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.P.K.; Chan, K.K.C.; Leung, H.W.; Huie, C.W. Pressurized liquid extraction of active ingredients (ginsenosides) from medicinal plants using non-ionic surfactant solutions. J. Chromatogr. A 2003, 983, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Aun, R.; Yan-Wei, W.; Xiu-Quan, X.; Guan-Hua, C. Optimization of Surfactant-Mediated, Ultrasonic-assisted Extraction of Antioxidant Polyphenols from Rattan Tea (Ampelopsis grossedentata) Using Response Surface Methodology. Pharmacogn. Mag. 2017, 13, 446–453. [Google Scholar] [Green Version]
- Zhang, X.; Ban, Q.; Wang, X.; Wang, Z. Green and Efficient PEG-Based Ultrasonic-Assisted Extraction of Polysaccharides from Tree Peony Pods and the Evaluation of Their Antioxidant Activity In Vitro. Biomed. Res. Int. 2018, 2018, 2121385. [Google Scholar] [CrossRef] [PubMed]
- Rezaeepour, R.; Heydari, R.; Ismaili, A. Ultrasound and salt-assisted liquid-liquid extraction as an efficient method for natural product extraction. Anal. Methods 2015, 7, 3253–3259. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Meza-Velázquez, J.A.; Simal, S.; Rossello, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Kara, N.; Erbas, D.; Baydar, H. The Effect of Seawater Used for Hydrodistillation on Essential Oil Yield and Composition of Oil-Bearing Rose ( Rosa damascena Mill.). Int. J. Sec. Metab. 2017, 4, 423–428. [Google Scholar]
- Charchari, S.; Abdelli, M. Enhanced Extraction by Hydrodistillation of Sage (Salvia officinalis L.) Essential Oil Using Water Solutions of Non-ionic Surfactants. J. Essent. Oil Bear. Plants 2015, 5026, 1094–1099. [Google Scholar]
- Hosseinzadeh, R.; Khorsandi, K.; Hemmaty, S. Study of the Effect of Surfactants on Extraction and Determination of Polyphenolic Compounds and Antioxidant Capacity of Fruits Extracts. PLoS ONE 2013, 8, e57353. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef]
- Krupa, A.; Majda, D.; Jachowicz, R.; Mozgawa, W. Solid-state interaction of ibuprofen and Neusilin US2. Thermochim. Acta 2010, 509, 12–17. [Google Scholar] [CrossRef]
- Sander, C.; Holm, P. Porous Magnesium Aluminometasilicate Tablets as Carrier of a Cyclosporine Self-Emulsifying Formulation. AAPS PharmSciTech 2009, 10, 1388. [Google Scholar] [CrossRef]
- Naiserova, M.; Kubova, K.; Vyslouzil, J.; Pavlokova, S.; Vetchy, D.; Urbanova, M.; Brus, J.; Vyslouzil, J.; Kulich, P. Investigation of Dissolution Behavior HPMC/Eudragit®/Magnesium Aluminometasilicate Oral Matrices Based on NMR Solid-State Spectroscopy and Dynamic Characteristics of Gel Layer. AAPS PharmSciTech 2017, 19, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.G.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.M.; Costa, J.; Casanova, J.; Bolla, J.M.; Berti, L. (E)-Methylisoeugenol and Elemicin: Antibacterial Components of Daucus carota L. Essential Oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Candido, A.G.F.; Wong, D.V.H. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Z.G.; Yu, P.J.; Wang, G.F.; Zhang, J.Y.; Li, J.R.; Ai, R.T.; Li, Z.H.; Tian, Y.X.; Zhang, W.X.; et al. Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-κB signalling pathway activation. Phyther. Res. 2012, 26, 1320–1326. [Google Scholar] [CrossRef]
- Lu, X.; Yang, L.; Chen, J.; Zhou, J.; Tang, X.; Zhu, Y.; Qiu, H.; Shen, J. The action and mechanism of myrislignan on A549 cells in vitro and in vivo. J. Nat. Med. 2017, 71, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Vanwert, A.; Bogner, R.H. Formation of Physically Stable Amorphous Drugs by Milling with Neusilin. J. Pharm. Sci. 2003, 92, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Khade, S.; Pore, Y. Formulation and Evaluation Of Neusilin® Us2 Adsorbed Amorphous Solid Self-Microemulsifying Delivery System of Atorvastatin Calcium. Asian J. Pharm. Clin. Res. 2016, 9, 93–100. [Google Scholar]
- Mihajilov-Krstev, T.; Jovanovic, B.; Jovic, J.; Ilic, B.; Miladinovic, D.; Matejic, J.; Rajkovic, J.; Dordevic, L.; Cvetkovic, V.; Zlatkovic, B. Antimicrobial, Antioxidative, and Insect Repellent Effects of Artemisia absinthium Essential Oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, J.; Zuzarte, M.; Goncalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. D-limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar] [PubMed]
- Giweli, A.; Dzamic, A.M.; Sokovic, M.; Ristic, M.S.; Marin, P.D. Antimicrobial and antioxidant activities of essential oils of satureja thymbra growing wild in libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef]
- Mallavarapu, G.R.; Ramesh, S. Composition of essential oils of nutmeg and mace. J. Med. Arom. Plant Sci 1998, 20, 746–748. [Google Scholar]
- Gupta, M. Pharmacological properties and traditional therapeutic uses of important indian spices: A review. Int. J. Food Prop. 2010, 13, 1092–1116. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical Composition of the Essential Oils of Aerial Parts and Flowers of Chromolaena odorata (L.) R. M. King & H. Rob. from Western Ghats Region of North West Karnataka, India. J. Essent. Oil-Bear. Plants 2013, 16, 71–75. [Google Scholar]
- Chu, S.; Liu, O.; Zhou, L.; Du, S.; Liu, Z. Chemical composition and toxic activity of essential oil of Caryopteris incana against Sitophilus zeamais. Afr. J. Biotechnol. 2013, 10, 8476–8480. [Google Scholar]
- Ekundayo, O.; Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A. Chemical composition of essential oil of myristica fragrans houtt (nutmeg) from nigeria. J. Essent. Oil-Bear. Plants 2003, 6, 21–26. [Google Scholar]
- Du, S.S.; Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Guo, S.S.; Deng, Z.W.; Liu, Z.L. Chemical constituents and activities of the essential oil from myristica fragrans against cigarette beetle lasioderma serricorne. Chem. Biodivers. 2014, 11, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |



| Method | Sample Code | Temperature | Time (h) | Solvent | Solvent: Nutmeg Ratio | Magnesium Aluminometasilicate: Nutmeg Ratio |
|---|---|---|---|---|---|---|
| Maceration | M1 | ambient | 72 | purified water | 20:1 | - |
| Ultrasound-Assisted Extraction | UAE1 | 25 °C | 0.5 | ethanol 50% | 20:1 | - |
| UAE2 | ethanol 70% | 20:1 | - | |||
| UAE3 | ethanol 96% | 20:1 | - | |||
| UAE4 | ethanol 70% + 0.5% magnesium aluminometasilicate | 20:1 | 10:1 | |||
| UAE5 | ethanol 70% + 1% magnesium aluminometasilicate | 20:1 | 5:1 | |||
| UAE6 | ethanol 70% + 2% magnesium aluminometasilicate | 20:1 | 2.5:1 | |||
| Hydrodistillation | HD1 | 100 °C | 4 | purified water | 20:1 | - |
| HD2 | purified water + 0.5% magnesium aluminometasilicate | 20:1 | 10:1 | |||
| HD3 | purified water + 1% magnesium aluminometasilicate | 20:1 | 5:1 | |||
| HD4 | purified water + 2% magnesium aluminometasilicate | 20:1 | 2.5:1 |
| Compound | Sample Code a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential Oil Compounds | Extracts Compounds | |||||||||||
| RI d | HD e 1 (%) | HD2 (%) | HD3 (%) | HD4 (%) | M f 1 (%) | UAE g 1 (%) | UAE2 (%) | UAE3 (%) | UAE4 (%) | UAE5 (%) | UAE6 (%) | |
| 4-Carene, trans -(+)- | 911 | 7.77 | - | - | - | 3.37 | 1.19 | 6.56 | 13.22 | - | - | - |
| α-Thujene | 928 | 0.99 | 1.04 | 0.93 | 0.99 | - | 2.63 | 0.72 | 1.42 | 0.84 | 0.92 | 0.81 |
| α-Pinene | 934 | 8.27 | 10.04 | 15.05 | 11.5 | - | 2.4 | 8.3 | 14.67 | 9.65 | 8.13 | 9.94 |
| Camphene | 944 | - | 2.8 | 0.11 | 0.11 | - | - | - | - | - | - | 0.15 |
| Orthodene b | 949 | 0.51 | - | - | - | - | - | - | 0.59 | - | - | - |
| 3-Carene | 956 | 0.53 | - | - | - | - | - | 0.42 | 0.78 | - | - | - |
| α-Phellandrene | 957 | - | - | - | - | 13.04 | - | 1.41 | 2.38 | - | - | - |
| β-Myrcene | 958 | - | - | - | - | 4.6 | - | - | - | 9.47 | 7.26 | 9.19 |
| 2-Carene | 960 | 1.99 | - | - | - | - | - | 0.75 | 1.15 | - | - | - |
| Sabinene | 969 | 6.53 | 49.64 | 61.42 | 47.1 | - | - | - | - | 29.9 | 31.74 | 32.69 |
| β-Pinene | 970 | 26.61 | 4.03 | 4.32 | 3.84 | - | - | - | - | - | - | - |
| Myristicin | 981 | 3.26 | 2.34 | 2.15 | 2.16 | - | - | - | - | 1.79 | 1.65 | 1.96 |
| γ-Terpinene | 993 | - | 0.78 | 0.68 | 0.73 | - | - | - | - | 0.68 | 1.65 | 0.71 |
| α-Terpinene | 999 | 1.23 | 1.23 | 0.94 | 1.23 | - | - | 0.6 | 1.07 | 0.67 | 1.18 | 0.69 |
| 3,7,7-trimethylcyclohepta-1,3,5-triene | 1005 | 0.18 | 0.47 | 0.33 | 0.44 | - | - | - | - | 0.37 | - | - |
| Limonene | 1009 | - | 5.62 | 4.2 | 5.23 | - | - | - | - | 4.2 | 4.46 | 4.63 |
| Isoterpinolene | 1030 | - | 2.11 | 1.18 | 2.07 | - | - | - | - | 1.19 | 2.02 | 1.22 |
| Cis-sabinen hydrate | 1037 | 7.76 | 0.58 | 0.3 | 0.83 | - | - | - | - | 1.3 | 2.32 | 1.46 |
| γ-Terpineol | 1043 | - | 0.04 | 0.04 | 0.04 | - | - | - | 0.14 | - | - | 0.09 |
| α-Terpinolene | 1051 | - | 0.7 | 0.38 | 0.7 | - | - | - | - | - | - | 0.54 |
| Sylvestrene | 1059 | - | - | - | - | 1.57 | - | - | - | - | - | - |
| Isomethyleugenol | 1062 | 0.2 | - | - | - | 6.38 | - | - | - | - | - | - |
| Cis-p-menth-2-en-1-ol | 1076 | 2.02 | 0.38 | 0.37 | 0.38 | 0.43 | 3.27 | 5.88 | 3.25 | 1.32 | 2.42 | 1.62 |
| 4-Propenyl syringol | 1083 | - | - | - | - | - | 2.16 | 1.55 | - | - | - | - |
| Citronellyl Decanoate b | 1110 | 0.3 | - | - | - | - | - | 0.43 | 0.16 | - | - | - |
| 1,1-dimethyl-2-[(1E)-3-methylbuta-1,3-dienyl cyclopropane | 1116 | - | - | - | - | - | 8.4 | 29.99 | 47.32 | - | - | - |
| Bicyclogermacrene | 1125 | 0.29 | - | - | - | - | - | - | - | - | - | - |
| 4-Terpineol | 1152 | 0.74 | 0.34 | 0.03 | 0.35 | - | - | 0.77 | 0.3 | 0.47 | 0.47 | 0.47 |
| Cubebol b | 1174 | 0.05 | - | - | - | - | - | - | - | - | - | - |
| Cubenene b | 1177 | 0.07 | - | - | - | - | - | - | - | - | - | - |
| Piperitol | 1189 | 0.09 | - | - | - | - | - | - | - | - | - | - |
| Isoelemicin | 1217 | 5.98 | - | - | - | - | 62.24 | 23.-63 | - | 21.5 | 21.5 | 18.85 |
| Copaene | 1228 | - | 0.25 | 0.01 | 0.25 | - | - | - | - | - | - | - |
| β-Copaene b | 1233 | 0.25 | - | - | - | - | - | - | - | - | - | - |
| γ-Amorphene b | 1245 | - | 0.75 | 0.03 | 0.78 | - | - | - | - | 0.43 | 0.92 | 0.08 |
| Cis-α-bergamotene b | 1283 | - | 0.09 | 0.08 | 0.1 | - | - | - | - | - | - | - |
| Isogermacrene | 1311 | - | 0.99 | 0.01 | 1.15 | 1.61 | - | - | - | 0.86 | 2.44 | 0.82 |
| Licarin B | 1328 | - | - | - | - | - | - | 0.72 | - | 0.71 | - | - |
| Bergamotene b | 1339 | 0.07 | - | - | - | - | - | - | - | - | - | - |
| γ-Asarone | 1361 | 3.69 | 1.21 | 1.22 | 3.51 | 0.79 | 0.84 | 2.07 | - | 0.82 | 1.84 | 1.25 |
| Elemicin | 1542 | - | - | - | - | 13.99 | - | - | - | - | - | - |
| Myrislignan | 2946 | - | - | - | - | 22.59 | - | - | - | - | - | - |
| Total Identified Compounds % c | 79.38 | 85.43 | 93.78 | 83.6 | 68.37 | 83.13 | 83.8 | 86.45 | 86.1 | 90.92 | 87.17 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalvėnienė, Z.; Lazauskas, R.; Bernatoniene, J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules 2019, 24, 1062. https://doi.org/10.3390/molecules24061062
Matulyte I, Marksa M, Ivanauskas L, Kalvėnienė Z, Lazauskas R, Bernatoniene J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules. 2019; 24(6):1062. https://doi.org/10.3390/molecules24061062
Chicago/Turabian StyleMatulyte, Inga, Mindaugas Marksa, Liudas Ivanauskas, Zenona Kalvėnienė, Robertas Lazauskas, and Jurga Bernatoniene. 2019. "GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient" Molecules 24, no. 6: 1062. https://doi.org/10.3390/molecules24061062
APA StyleMatulyte, I., Marksa, M., Ivanauskas, L., Kalvėnienė, Z., Lazauskas, R., & Bernatoniene, J. (2019). GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules, 24(6), 1062. https://doi.org/10.3390/molecules24061062