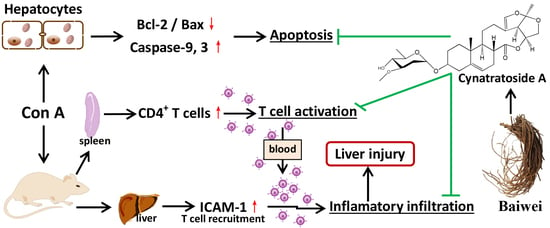
A C21-Steroidal Glycoside from Cynanchum atratum Attenuates Concanavalin A-Induced Liver Injury in Mice
Abstract
:1. Introduction
2. Results
2.1. CyA Inhibits Con A-Induced Pathological Changes of Spleen in Mice
2.2. CyA Suppresses T Lymphocyte Proliferation in the Spleen of Con A-Induced Mice
2.3. CyA Attenuates the Expression of IL-1β and ICAM-1 in the Liver of Con A-Injured Mice
2.4. CyA Alleviates Con A-Induced Liver Injury in Mice
2.5. CyA Protects Hepatocytes from Con A-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Con A-Induced AIH Model and Drug Treatment Protocols in Mice
4.4. Detection of Biochemical Indexes
4.5. Histopathological and Immunohistochemical Examination
4.6. Flow Cytometry Analysis of CD4+ and CD8+ Cells
4.7. Cell Culture and Treatment
4.8. Determination of Cell Viability
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIH | autoimmune hepatitis |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| Bax | BCL2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| CC50 | median cytotoxic concentration |
| Con A | concanavalin A |
| CyA | cynatratoside A |
| IC50 | half maximal inhibitory concentration |
| ICAM-1 | intercellular adhesion molecule-1 |
| IOD | integrated optical density |
| IL-1β | interleukin-1β |
| KCs | Kupffer cells |
| LDH | lactic dehydrogenase |
| MHC | major histocompatibility complex |
| MTT | methyl thiazolyl tetrazolium |
| SECs | sinusoid endothelial cells |
| TBil | total bilirubin |
References
- Bertolino, P.; Klimpel, G.; Lemon, S.M. Hepatic inflammation and immunity: A summary of a conference on the function of the immune system within the liver. Hepatology 2000, 31, 1374–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Baird, A.W.; O’Farrelly, C. Microanatomy of the liver immune system. Semin. Immunopathol. 2009, 31, 333. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.G. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J. Autoimmun. 2016, 66, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, P.J.; Roberts, T.; Sands, A.; Peeling, R. Diagnosis of viral hepatitis. Curr. Opin. HIV AIDS 2017, 12, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Liwinski, T.; Schramm, C. Autoimmune hepatitis - update on clinical management in 2017. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 617–625. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Zachou, K.; Koukoulis, G.K.; Dalekos, G.N. Autoimmune hepatitis, one disease with many faces: Etiopathogenetic, clinico-laboratory and histological characteristics. World J. Gastroenterol. 2015, 21, 60–83. [Google Scholar] [CrossRef]
- van Gerven, N.M.; de Boer, Y.S.; Mulder, C.J.; van Nieuwkerk, C.M.; Bouma, G. Auto immune hepatitis. World J. Gastroenterol. 2016, 22, 4651–4661. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Wu, Z.F.; Yu, S.L.; Wang, L.; Ye, W.C.; Zhang, Q.W.; Yin, Z.Q. Cytotoxic and apoptosis-inducing activity of C21 steroids from the roots of Cynanchum atratum. Steroids 2017, 122, 1–8. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Lee, H.; Ahn, K.S.; Um, J.Y.; Lee, S.G.; Kim, J.; Yang, W.M. Cynanchum atratum inhibits the development of atopic dermatitis in 2,4-dinitrochlorobenzene-induced mice. Biomed. Pharmacother. 2017, 90, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, J.X.; Liu, K.X.; Huang, T.; Yan, C.; Huang, L.J.; Liu, S.; Mu, S.Z.; Hao, X.J. Seco-pregnane steroidal glycosides from the roots of Cynanchum atratum and their anti-TMV activity. Fitoterapia 2014, 97, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.W.; Zhang, Q.Z.; Xu, D.H.; Liang, J.H.; Wang, B. Antiparasitic effect of cynatratoside-C from Cynanchum atratum against Ichthyophthirius multifiliis on grass carp. J. Agric. Food Chem. 2014, 62, 7183–7189. [Google Scholar] [CrossRef]
- Jin, Q.; Han, X.H.; Yun, C.Y.; Lee, C.; Lee, J.W.; Lee, D.; Lee, M.K.; Jung, S.H.; Hong, J.T.; Kim, Y.; et al. Melanogenesis inhibitory pregnane glycosides from Cynanchum atratum. Bioorg. Med. Chem. Lett. 2018, 28, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Ding, M.L.; Tao, L.J.; Zhang, M.; Xu, X.H.; Zhang, C.F. Immunosuppressive C21 steroidal glycosides from the root of Cynanchum atratum. Fitoterapia 2015, 105, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Son, S.W.; Kim, H.G.; Han, J.M.; Lee, J.S.; Choi, M.K.; Lee, J.S.; Son, C.G. Anti-melanoma activity of Cynanchi Atrati Radix is mediated by regulation of NF-kappa B activity and pro-apoptotic proteins. J. Ethnopharmacol. 2014, 153, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Qin, J.J.; Chen, Z.H.; Zhou, Y.; Tang, W.; Zuo, J.P.; Zhao, W.M. Absolute configuration of periplosides C and F and isolation of minor spiro-orthoester group-containing pregnane-type steroidal glycosides from Periploca sepium and their T-lymphocyte proliferation inhibitory activities. J. Nat. Prod. 2017, 80, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, X.; Yang, Y.; Yang, Y.; Shi, S.; Guo, F.; Li, Y. Sixteen novel C-21 steroidal glycosides from the roots of Cynanchum mooreanum. J. Nat. Prod. 2015, 104, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Ye, Y.; Chen, F.; Pan, Y. C-21 steroidal glycosides from the roots of Cynanchum chekiangense and their immunosuppressive activities. Steroids 2006, 71, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Liu, M.; Weng, S.Y.; Li, J.J.; Xie, C.; He, H.L.; Guan, W.; Yuan, Y.S.; Gao, J. Immune mechanisms of concanavalin A model of autoimmune hepatitis. World J. Gastroenterol. 2012, 18, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T-cell-dependent experimental liver-injury in mice inducible by concanavalin-A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Kido, M.; Iwamoto, S.; Nishiura, H.; Maruoka, R.; Tanaka, J.; Watanabe, T.; Tanaka, Y.; Okazaki, T.; Chiba, T.; et al. Dysregulated generation of follicular helper T cells in the spleen triggers fatal autoimmune hepatitis in mice. Gastroenterology 2011, 140, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, A.; Surdacka, A.; Daniluk, J. Bcl-2 and Fas expression in peripheral blood leukocytes of patients with alcoholic and autoimmune liver disorders. Hum. Exp. Toxicol. 2016, 35, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Liu, T.; Tan, W.; Ren, H.; Li, H.; Liu, J.; Cao, H.; Cheng, Q.; Liu, X.; Zhu, H.; et al. Effects of kinsenoside, a potential immunosuppressive drug for autoimmune hepatitis, on dendritic cells/CD8(+) T cells communication in mice. Hepatology 2016, 64, 2135–2150. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, Y.; Aoki, C.A.; Bowlus, C.L.; Shimoda, S.; Ishibashi, H.; Gershwin, M.E. T cell immunity in autoimmune hepatitis. Autoimmun. Rev. 2005, 4, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, G.; Tsunematsu, S.; Tsukinoki, K.; Ohmi, Y.; Iwamiya, M.; Oliveira-dos-Santos, A.; Tone, D.; Shindo, J.; Penninger, J.M. Essential role of the adhesion receptor LFA-1 for T cell-dependent fulminant hepatitis. J. Immunol. 2002, 169, 7087–7096. [Google Scholar] [CrossRef]
- Proudman, S.M.; Cleland, L.G.; Mayrhofer, G. Effects of tumor necrosis factor-alpha, interleukin 1beta, and activated peripheral blood mononuclear cells on the expression of adhesion molecules and recruitment of leukocytes in rheumatoid synovial xenografts in SCID mice. J. Rheumatol. 1999, 26, 1877–1889. [Google Scholar]
- Sun, Q.; Xu, X.; Yang, X.; Weng, D.; Wang, J.; Zhang, J. Salecan protected against concanavalin A-induced acute liver injury by modulating T cell immune responses and NMR-based metabolic profiles. Toxicol. Appl. Pharmacol. 2017, 317, 63–72. [Google Scholar] [CrossRef]
- Sadeghi, H.; Lockmann, A.; Hund, A.C.; Samavedam, U.K.; Pipi, E.; Vafia, K.; Hauenschild, E.; Kalies, K.; Pas, H.H.; Jonkman, M.F.; et al. Caspase-1-independent IL-1 release mediates blister formation in autoantibody-induced tissue injury through modulation of endothelial adhesion molecules. J. Immunol. 2015, 194, 3656–3663. [Google Scholar] [CrossRef]
- Leist, M.; Wendel, A. A novel mechanism of murine hepatocyte death inducible by Concanavalin A. J. Hepatol. 1996, 25, 948–959. [Google Scholar] [CrossRef]
- Watanabe, Y.; Morita, M.; Akaike, T. Concanavalin A induces perforin-mediated but not Fas-mediated hepatic injury. Hepatology 1996, 24, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Wang, C.; Liu, Q.; Yang, T.; Zhang, Q.; Peng, J.; Gao, Y.; Sun, H.; Kaku, T.; Liu, K. Protective effect of JBP485 on concanavalin A-induced hepatocyte toxicity in primary cultured rat hepatocytes. Eur. J. Pharmacol. 2008, 589, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Niu, P.; Chen, K.; Wu, L.; Liu, T.; Xu, S.; Li, J.; Li, S.; Wang, W.; Lu, X.; et al. Salidroside mediates apoptosis and autophagy inhibition in concanavalin A-induced liver injury. Exp. Ther. Med. 2018, 15, 4599–4614. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, J.; Yu, F.; Cheng, J.; Li, H.; Guo, C.; Fan, X. Ghrelin reduces liver impairment in a model of concanavalin A-induced acute hepatitis in mice. Drug Des. Dev. Ther. 2015, 9, 5385–5396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Xia, Z.; Yu, D.; Wang, J.; Jin, L.; Huang, D.; Ye, X.; Li, X.; Zhang, B. Hepatoprotective effects and structure-activity relationship of five flavonoids against lipopolysaccharide/d-galactosamine induced acute liver failure in mice. Int. Immunopharmacol. 2019, 68, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Rlloyd, A. Chemokines: Leucocyte recruitment and activation cytokines. Lancet 1997, 349, 490–495. [Google Scholar] [CrossRef]
- Muller, W.A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003, 24, 327–334. [Google Scholar] [CrossRef]
- Wuthrich, R.P.; Jevnikar, A.M.; Takei, F.; Glimcher, L.H.; Kelley, V.E. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am. J. Pathol. 1990, 136, 441–450. [Google Scholar]
- Cuzzocrea, S.; Crisafulli, C.; Mazzon, E.; Esposito, E.; Muia, C.; Abdelrahman, M.; Di Paola, R.; Thiemermann, C. Inhibition of glycogen synthase kinase-3beta attenuates the development of carrageenan-induced lung injury in mice. Br. J. Pharmacol. 2006, 149, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, C.Y.; Bian, H.J.; Min, M.W.; Chen, L.F.; Bao, J.K. Antiproliferative activity and apoptosis-inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 2009, 482, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Ding, X.; Yang, Y. In silico and experimental studies of concanavalin A: Insights into its antiproliferative activity and apoptotic mechanism. Appl. Biochem. Biotechnol. 2010, 162, 134–145. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound is not available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, B.; Zhang, C.-f.; Xu, X.-h.; Zhang, M. A C21-Steroidal Glycoside from Cynanchum atratum Attenuates Concanavalin A-Induced Liver Injury in Mice. Molecules 2019, 24, 1087. https://doi.org/10.3390/molecules24061087
Yang J, Wang B, Zhang C-f, Xu X-h, Zhang M. A C21-Steroidal Glycoside from Cynanchum atratum Attenuates Concanavalin A-Induced Liver Injury in Mice. Molecules. 2019; 24(6):1087. https://doi.org/10.3390/molecules24061087
Chicago/Turabian StyleYang, Jian, Bin Wang, Chao-feng Zhang, Xiang-hong Xu, and Mian Zhang. 2019. "A C21-Steroidal Glycoside from Cynanchum atratum Attenuates Concanavalin A-Induced Liver Injury in Mice" Molecules 24, no. 6: 1087. https://doi.org/10.3390/molecules24061087
APA StyleYang, J., Wang, B., Zhang, C. -f., Xu, X. -h., & Zhang, M. (2019). A C21-Steroidal Glycoside from Cynanchum atratum Attenuates Concanavalin A-Induced Liver Injury in Mice. Molecules, 24(6), 1087. https://doi.org/10.3390/molecules24061087