Systematically Characterize the Anti-Alzheimer’s Disease Mechanism of Lignans from S. chinensis Based on In-Vivo Ingredient Analysis and Target-Network Pharmacology Strategy by UHPLC–Q-TOF-MS
Abstract
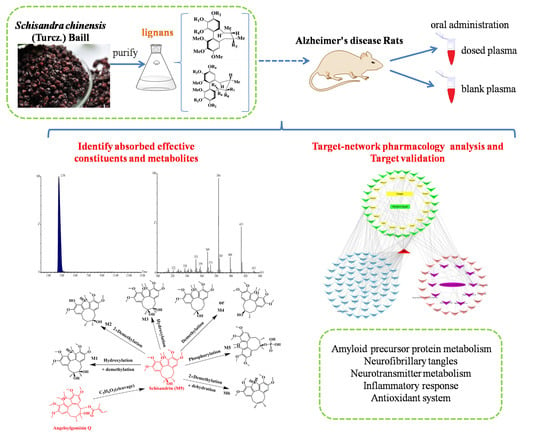
:1. Introduction
2. Results
2.1. Identification of Absorbed Effective Constituents and their Metabolites from Lignans in S. chinensis
2.2. Target Genes Related to the Identified Compounds
2.3. Target Validation
2.3.1. Effects of Lignans from S. chinensis on Aβ Deposition, p-tau Levels, and Number of Neurons in the Hippocampus of Rats with AD
2.3.2. Effects of Lignans from S. chinensis on Inflammatory and Oxidant Damage in Rats with AD
2.3.3. Effects of Lignans from S. chinensis on Neurotransmitters in Rats with AD
2.3.4. Effects of Lignans from S. chinensis on other Pathways in Rats with AD
3. Discussion
3.1. Effects of Lignans from S. chinensis on the Aβ and p-tau Levels, and Hippocampal Neuronal Morphology in Rats with AD
3.2. Effects of Lignans from S. chinensis on Inflammatory and Oxidant Damage in Rats with AD
3.3. Effects of Lignans from S. chinensis on Neurotransmitters in Rats with AD
3.4. Effects of Lignans from S. chinensis on other Pathways in Rats with AD
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Establishment of the AD Model and Drug Administration
4.3. Effective Constituents Absorbed in the Blood
4.3.1. Plasma Sample Collection and Preparation
4.3.2. UHPLC–MS Conditions and Data Processing of Plasma Analysis
4.4. Network Construction and Pathway Analyses
4.5. Target Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Li, Z.; Tong, Q.; Xu, H.; Hu, L.; Zhao, R.; Zhou, F.; Pan, W.; Zhou, L. Therapeutic Effects of TianDiJingWan on the Aβ 25-35-Induced Alzheimer’s Disease Model Rats. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; De, B.S. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698. [Google Scholar] [CrossRef]
- Monacelli, F.; Rosa, G. Cholinesterase inhibitors: Cardioprotection in Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, 1071–1077. [Google Scholar] [CrossRef]
- Mattace-Raso, F. Is memantine + acetylcholinesterase inhibitor treatment superior to either therapy alone in Alzheimer’s disease? J. Alzheimers Dis. 2014, 41, 641–642. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. J. Pharm. Anal. 2012, 70, 451–471. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Han, W.; Han, H.; Liu, Y.; Guan, W.; Kuang, H. Lignans from Schisandra chinensis rattan stems suppresses primary Abeta1-42-induced microglia activation via NF-kappaB/MAPK signaling pathway. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Yan, T.; Xu, M.; Wu, B.; Liao, Z.; Liu, Z.; Zhao, X.; Bi, K.; Jia, Y. The effect of Schisandra chinensis extracts on depression by noradrenergic, dopaminergic, GABAergic and glutamatergic systems in the forced swim test in mice. Food Funct. 2016, 7, 2811–2819. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, H.K.; Lee, K.Y.; Jeon, B.J.; Kim, D.H.; Park, J.-H.; Song, J.-H.; Huh, J.; Lee, J.-H.; Sung, S.H. The effects of lignan-riched extract of Shisandra chinensis on amyloid-β-induced cognitive impairment and neurotoxicity in the cortex and hippocampus of mouse. J. Ethnopharmacol. 2013, 146, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Shang, L.; Wang, M.; Zhang, C.; Zhao, X.; Bi, K.; Jia, Y. Lignans from Schisandra chinensis ameliorate cognition deficits and attenuate brain oxidative damage induced by D-galactose in rats. Metab. Brain Dis. 2016, 31, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Abeta1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLoS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef]
- Zifeng, P.; Qianqian, W.; Jing, Z.; Fengrui, S.; Zhiqiang, L. Effect of Schisandra Fruit on Neurochemicals in Hippocampus of Diabetic Encephalopathy Rat Using Online MD-HPLC-MS/MS. Chem. J. Chin. Univ. 2015, 36, 442–448. [Google Scholar] [CrossRef]
- Sun, H.; Wu, F.; Zhang, A.; Wei, W.; Han, Y.; Wang, X. Profiling and identification of the absorbed constituents and metabolites of schisandra lignans by ultra-performance liquid chromatography coupled to mass spectrometry. Biomed. Chromatogr. 2013, 27, 1511–1519. [Google Scholar] [CrossRef]
- Lou, Z.; Zhang, H.; Gong, C.; Zhu, Z.; Zhao, L.; Xu, Y.; Wang, B.; Zhang, G. Analysis of lignans in Schisandra chinensis and rat plasma by high-performance liquid chromatography diode-array detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 831–842. [Google Scholar] [CrossRef]
- Shao, L.I.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Xu, T.; Li, S.; Sun, Y.; Pi, Z.; Liu, S.; Song, F.; Liu, Z. Systematically characterize the absorbed effective substances of Wutou Decoction and their metabolic pathways in rat plasma using UHPLC-Q-TOF-MS combined with a target network pharmacological analysis. J. Pharm. Biomed. Anal. 2017, 141, 95. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Song, Y.; Liu, Y.; Peng, M.; Li, H.; Li, X.; Feng, B.; Xu, P.; Su, D. An integrated strategy using UPLC–QTOF-MS E and UPLC–QTOF-MRM (enhanced target) for pharmacokinetics study of wine processed Schisandra Chinensis fructus in rats. J. Pharm. Biomed. Anal. 2017, 139, 165–178. [Google Scholar] [CrossRef]
- Shilong, M.; Hai, Z.; Lei, L.; Zhenyu, Z.; Liang, Z.; Guoqing, Z.; Yifeng, C. Rapid determination and pharmacokinetics study of lignans in rat plasma after oral administration of Schisandra chinensis extract and pure deoxyschisandrin. Biomed. Chromatogr. 2011, 25, 808–815. [Google Scholar] [CrossRef]
- Deng, L.; Shi, A.M.; Liu, H.Z.; Meruva, N.; Liu, L.; Hu, H.; Yang, Y.; Huang, C.; Li, P.; Wang, Q. Identification of chemical ingredients of peanut stems and leaves extracts using UPLC-QTOF-MS coupled with novel informatics UNIFI platform. J. Mass Spectrom. 2016, 51, 1157–1167. [Google Scholar] [CrossRef]
- Minyan, L.; Shaohua, Z.; Zongquan, W.; Yufeng, W.; Ting, L.; Song, L.; Cuicui, W.; Hongtao, W.; Pengfei, T. Identification of metabolites of deoxyschizandrin in rats by UPLC-Q-TOF-MS/MS based on multiple mass defect filter data acquisition and multiple data processing techniques. J. Chromatogr. Anal. Technol. Biomed. Life Sci. 2014, 949–950, 115–126. [Google Scholar] [CrossRef]
- Hua, W.; Haijun, M.; Yunlei, Y.; Jingxian, L.; Xiaofeng, Q.; Rong, W.; Wansheng, C. Validation of an LC-MS/MS method for quantitative analysis of the 5 bioactive components of Wuzhi capsule in human plasma samples. Ther. Drug Monit. 2014, 36, 781–788. [Google Scholar] [CrossRef]
- Steele, M.; Stuchbury, G.; Münch, G. The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp. Gerontol. 2007, 42, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Greenamyre, J.T.; Young, A.B. Excitatory amino acids and Alzheimer’s disease. Neurobiol. Aging 1989, 10, 593–602. [Google Scholar] [CrossRef]
- Szot, P.; Franklin, A.; Miguelez, C.; Wang, Y.; Vidaurrazaga, I.; Ugedo, L.; Sikkema, C.; Wilkinson, C.W.; Raskind, M.A. Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine. Neuropharmacology 2016, 101, 76–86. [Google Scholar] [CrossRef]
- Trillo, L.; Das, D.; Hsieh, W.; Medina, B.; Moghadam, S.; Lin, B.; Dang, V.; Sanchez, M.M.; De, M.Z.; Ashford, J.W. Ascending monoaminergic systems alterations in Alzheimer’s disease. translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013, 37, 1363–1379. [Google Scholar] [CrossRef]
- Siddique, H.; Hynan, L.S.; Weiner, M.F. Effect of a serotonin reuptake inhibitor on irritability, apathy, and psychotic symptoms in patients with Alzheimer’s disease. J. Clin. Psychiatry 2009, 70, 915–918. [Google Scholar] [CrossRef]
- Cho, J.H.; Johnson, G.V. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J. Neurochem. 2004, 88, 349–358. [Google Scholar] [CrossRef]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, S141–S144. [Google Scholar] [CrossRef] [PubMed]
- Akimitsu, N.; Adachi, N.; Hirai, H.; Hossain, M.S.; Hamamoto, H.; Kobayashi, M.; Aratani, Y.; Koyama, H.; Sekimizu, K. Enforced cytokinesis without complete nuclear division in embryonic cells depleting the activity of DNA topoisomerase IIα. Genes Cells 2003, 8, 393–402. [Google Scholar] [CrossRef]
- Sapetto-Rebow, B.; McLoughlin, S.C.; O’Shea, L.C.; O’Leary, O.; Willer, J.R.; Alvarez, Y.; Collery, R.; O’Sullivan, J.; Van Eeden, F.; Hensey, C. Maternal topoisomerase II alpha, not topoisomerase II beta, enables embryonic development of zebrafish top2a-/-mutants. BMC Dev. Biol. 2011, 11, 71. [Google Scholar] [CrossRef]
- Heng, X.; Le, W.-D. The function of DNA topoisomerase IIβ in neuronal development. Neurosci. Bull. 2010, 26, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Nur-E-Kamal, A.; Meiners, S.; Ahmed, I.; Azarova, A.; Lin, C.-P.; Lyu, Y.L.; Liu, L.F. Role of DNA topoisomerase IIβ in neurite outgrowth. Brain Res. 2007, 1154, 50–60. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Burger, L.; Nikoletopoulou, V.; Deogracias, R.; Thakurela, S.; Wirbelauer, C.; Kaut, J.; Terranova, R.; Hoerner, L.; Mielke, C. Target genes of Topoisomerase IIβ regulate neuronal survival and are defined by their chromatin state. Proc. Natl. Acad. Sci. USA 2012, 109, E934–E943. [Google Scholar] [CrossRef]
- Bilen, J.; Liu, N.; Burnett, B.G.; Pittman, R.N.; Bonini, N.M. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol. Cell 2006, 24, 157–163. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef]
- Liu, N.; Landreh, M.; Cao, K.; Abe, M.; Hendriks, G.-J.; Kennerdell, J.R.; Zhu, Y.; Wang, L.-S.; Bonini, N.M. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature 2012, 482, 519. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Breunig, J.J.; Depboylu, C.; Rouaux, C.; Michel, P.P.; Alvarez-Fischer, D.; Boutillier, A.-L.; DeGregori, J.; Oertel, W.H.; Rakic, P.; et al. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Osuga, H.; Osuga, S.; Wang, F.; Fetni, R.; Hogan, M.J.; Slack, R.S.; Hakim, A.M.; Ikeda, J.-E.; Park, D.S. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 2000, 97, 10254–10259. [Google Scholar] [CrossRef] [PubMed]
- Busser, J.; Geldmacher, D.S.; Herrup, K. Ectopic Cell Cycle Proteins Predict the Sites of Neuronal Cell Death in Alzheimer’s Disease Brain. J. Neurosci. 1998, 18, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.W.; Mattson, M.P.; Wong, P.C.; Gleichmann, M. An overview of APP processing enzymes and products. Neuromol. Med. 2010, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.P.; Heneka, M.T. Truncated and modified amyloid-beta species. Alzheimers Res. Ther. 2014, 6, 28. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Shin, D.; Downs, D.; Koelsch, G.; Lin, X.; Ermolieff, J.; Tang, J. Design of potent inhibitors for human brain memapsin 2 (β-secretase). J. Am. Chem. Soc. 2000, 122, 3522–3523. [Google Scholar] [CrossRef]
- Dawson, T.M.; Snyder, S.H. Gases as biological messengers: Nitric oxide and carbon monoxide in the brain. J. Neurosci. 1994, 14, 5147–5159. [Google Scholar] [CrossRef]
- Giraldi-Guimarães, A.; Bittencourt-Navarrete, R.; Mendez-Otero, R. Expression of neuronal nitric oxide synthase in the developing superficial layers of the rat superior colliculus. Braz. J. Med. Biol. Res. 2004, 37, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.-G.; Bogerts, B.; Keilhoff, G. The many faces of nitric oxide in schizophrenia. A review. Schizophr. Res. 2005, 78, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Koskinen, A.; Kukkonen, M.; Nieminen, R.; Paivarinta, U.; Moilanen, T.; Moilanen, E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Med. Inflamm. 2009, 2009, 345838. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef]
- Kim, S.F.; Huri, D.A.; Snyder, S.H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005, 310, 1966–1970. [Google Scholar] [CrossRef] [PubMed]
- Pompeia, C.; Lima, T.R. Arachidonic acid cytotoxicity: Can arachidonic acid be a physiological mediator of cell death? Cell Biochem. Funct. 2010, 21, 97–104. [Google Scholar] [CrossRef]
- Ruparel, S.; Green, D.; Chen, P.; Hargreaves, K.M. The cytochrome P450 inhibitor, ketoconazole, inhibits oxidized linoleic acid metabolite-mediated peripheral inflammatory pain. Mol. Pain 2012, 8, 73. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Ikegami, T.; Iwamoto, J.; Maeda, T.; Hirayama, T.; Saito, Y.; Teramoto, T.; Matsuzaki, Y. Cholesterol 25-hydroxylation activity of CYP3A. J. Lipid Res. 2011, 52, 1509–1516. [Google Scholar] [CrossRef]
- Shimshoni, J.A.; Roberts, A.G.; Scian, M.; Topletz, A.R.; Blankert, S.A.; Halpert, J.R.; Nelson, W.L.; Isoherranen, N. Stereoselective Formation and Metabolism of 4-Hydroxy-Retinoic Acid Enantiomers by Cytochrome P450 Enzymes. J. Biol. Chem. 2012, 287, 42223–42232. [Google Scholar] [CrossRef]
- Pahl, A. Thromboxane-A Synthase. Xpharm Compr. Pharm. Ref. 2008, 1–6. [Google Scholar] [CrossRef]
- Li, W.-L.; Xin, H.-W.; Yu, A.-R.; Wu, X.-C. In vivo effect of Schisandrin B on cytochrome P450 enzyme activity. Phytomedicine 2013, 20, 760–765. [Google Scholar] [CrossRef]
- Li, W.L.; Xin, H.W.; Su, M.W. Inhibitory effects of continuous ingestion of Schisandrin A on CYP3A in the rat. Basic Clin. Pharmacol. Toxicol. 2012, 110, 187–192. [Google Scholar] [CrossRef]
- Li, W.L.; Xin, H.W.; Su, M.W.; Xiong, L. Inhibi ory effects of schisandrin A and schisandrin B on CYP3A activity. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H.; Si, Y.; Hu, D.; Yu, Y.; Zhang, Y.; Gao, M.; Zhang, H. Iodine Promotes Tumorigenesis of Thyroid Cancer by Suppressing Mir-422a and Up-Regulating MAPK1. Cell. Physiol. Biochem. 2017, 43, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, D.; Zhang, L.; Lian, G.; Zhao, S.; Wang, C.; Yin, J.; Wu, C.; Yang, J. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway. Food Chem. Toxicol. 2014, 63, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Galimberti, A.; Maggioni, D.; Ravasi, M.; Pasini, S.; Nicolini, G.; Bossi, M.; Miloso, M.; Cavaletti, G.; Tredici, G. Role of MAPKs in platinum-induced neuronal apoptosis. Neurotoxicology 2009, 30, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bean, L.A.; Rani, A.; Jackson, T.; Foster, T.C. Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice. Hippocampus 2015, 25, 1556–1566. [Google Scholar] [CrossRef]
- Cnubben, N.H.; Rietjens, I.M.; Wortelboer, H.; van Zanden, J.; van Bladeren, P.J. The interplay of glutathione-related processes in antioxidant defense. Environ. Toxicol. Pharmacol. 2001, 10, 141–152. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Lim, J.H.; Lee, C.; Choi, H.C.; Woo, C.-H. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J. Biol. Chem. 2012, 287, 40722–40731. [Google Scholar] [CrossRef]
- Lee, T.-S.; Chau, L.-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240. [Google Scholar] [CrossRef]
- Takahashi, M.; Doré, S.; Ferris, C.D.; Tomita, T.; Sawa, A.; Wolosker, H.; Borchelt, D.R.; Iwatsubo, T.; Kim, S.-H.; Thinakaran, G. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron 2000, 28, 461–473. [Google Scholar] [CrossRef]
- Ko, K.M.; Lam, B.Y. Schisandrin B protects against tert-butylhydroperoxide induced cerebral toxicity by enhancing glutathione antioxidant status in mouse brain. Mol. Cell Biochem. 2002, 238, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; And, M.V.M.; Inze, D. Superoxide Dismutase and Stress Tolerance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Chen, Y.; Chong-Yu, X.U.; Wang, P.; Cai, Y.L.; Hua-Song, M.A. Effects of Long-Term Simulated Microgravity on Oxidant and Antioxidant Values in the Plasma and Lung Tissues of Rhesus Macaque. Environ. Energy Earth Sci. 2016, 2, 1. [Google Scholar] [CrossRef]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Myagkova, M.A.; Gavrilova, S.I.; Lermontova, N.N.; Kalyn, Y.B.; Selezneva, N.D.; Zharikov, G.A.; Kolykhalov, I.V.; Abramenko, T.V.; Serkova, T.P.; Bachurin, S.O. Autoantibodies to β-Amyloid and Neurotransmitters in Patients with Alzheimer’s Disease and Senile Dementia of the Alzheimer Type. Bull. Exp. Biol. Med. 2001, 131, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, D.; Udayabanu, M.; Nair, R.U.; Aneja, R.; Katyal, A. Benzamide protects delayed neuronal death and behavioural impairment in a mouse model of global cerebral ischemia. Behav. Brain Res. 2008, 192, 178–184. [Google Scholar] [CrossRef]
- Hou, L.N.; Xu, J.R.; Zhao, Q.N.; Gao, X.L.; Cui, Y.Y.; Xu, J.; Wang, H.; Chen, H.Z. A New Motif in the N-Terminal of Acetylcholinesterase Triggers Amyloid-β Aggregation and Deposition. CNS Neurosci. Ther. 2014, 20, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, H.Y.; Zhang, X.J. Increased expression of intranuclear AChE involved in apoptosis of SK-N-SH cells. Neurosci. Res. 2002, 42, 261–268. [Google Scholar] [CrossRef]
- Schmitt, J.A.; Wingen, M.; Ramaekers, J.G.; Evers, E.A.; Riedel, W.J. Serotonin and human cognitive performance. Curr. Pharm. Des. 2006, 12, 2473–2486. [Google Scholar] [CrossRef]
- Brucato, F.H.; Levin, E.D.; Mott, D.D.; Lewis, D.V.; Wilson, W.A.; Swartzwelder, H.S. Hippocampal long-term potentiation and spatial learning in the rat: Effects of GABAB receptor blockade. Neuroscience 1996, 74, 331. [Google Scholar] [CrossRef]
- Yin, Y.; Yuan, H.; Wang, C.; Pattabiraman, N.; Rao, M.; Pestell, R.G.; Glazer, R.I. 3-Phosphoinositide-dependent protein kinase-1 activates the peroxisome proliferator-activated receptor-γ and promotes adipocyte differentiation. Mol. Endocrinol. 2006, 20, 268–278. [Google Scholar] [CrossRef]
- Mukherjee, R.; Jow, L.; Croston, G.E.; Paterniti, J.R. Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARγ2 versus PPARγ1 and activation with retinoid X receptor agonists and antagonists. J. Biol. Chem. 1997, 272, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Pandareesh, M.D.; Kandikattu, H.K.; Razack, S.; Narayanappa, A.; Choudhari, R.; Vikram, A.; Doddapattar, P. Nutrition and Nutraceuticals in Neuroinflammatory and Brain Metabolic Stress: Implications for Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, M.; Yue, K.; Hu, M.; Li, S.; Men, L.; Pi, Z.; Liu, Z.; Liu, Z. Study on Urine Metabolic Profile of Abeta25-35-Induced Alzheimer’s Disease Using UHPLC-Q-TOF-MS. Neuroscience 2018, 394, 30–43. [Google Scholar] [CrossRef]
- Huang, X.; Song, F.; Liu, Z.; Liu, S. Studies on lignan constituents from Schisandra chinensis (Turcz.) Baill. fruits using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. J. Mass Spectr. 2007, 42, 1148–1161. [Google Scholar] [CrossRef]
- Shi, P.; He, Q.; Zhang, Y.; Qu, H.; Cheng, Y. Characterisation and identification of isomeric dibenzocyclooctadiene lignans from Schisandra Chinensis by high-performance liquid chromatography combined with electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2009, 20, 197–206. [Google Scholar] [CrossRef]
- He, X.G.; Lian, L.Z.; Lin, L.Z. Analysis of lignan constituents from Schisandra chinensis by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. 1997, 757, 81–87. [Google Scholar] [CrossRef]
- Kim, H.W.; Shin, J.H.; Lee, M.K.; Jang, G.H.; Lee, S.H.; Jang, H.H.; Jeong, S.T.; Kim, J.B. Qualitative and quantitative analysis of dibenzocyclooctadiene lignans for the fruits of Korean ‘Omija’(Schisandra chinensis). Korean J. Med. Crop Sci. 2015, 23, 385–394. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Z.L.; Chen, Y.Z. TTD: Therapeutic target database. NAR 2002, 30, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, M.; Gilson, M.K. Bindingdb: A web-accessible molecular recognition database. Comb. Chem. High Throughput Screen. 2001, 4, 719. [Google Scholar] [CrossRef]
- Joanna, A.; Bocchini, C.A.; Scott, A.F.; Ada, H. Mckusick’s online mendelian inheritance in man (omim). NAR 2009, 37, 793–796. [Google Scholar] [CrossRef]
- Michael, K.; Christian, V.M.; Monica, C.; Lars Juhl, J.; Peer, B. Stitch: Interaction networks of chemicals and proteins. NAR 2008, 36, 684–688. [Google Scholar] [CrossRef]
- Ru, J.; Peng, L.; Wang, J.; Wei, Z.; Li, B.; Chao, H.; Li, P.; Guo, Z.; Tao, W.; Yang, Y. Tcmsp: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Kanehisa, M. Using the kegg database resource. Curr. Protoc. Bioinform. 2012. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. David bioinformatics resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. NAR 2007. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Fang, Z.; Zhang, M.; Yi, Z.; Wen, C.; Shi, T. TCMID: Traditional chinese medicine integrative database for herb molecular mechanism analysis. NAR 2013, 41, D1089. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef] [PubMed]










| No. | Parent Compound | Rt (min) | Measured Mass | Formula | Time Points (min) |
|---|---|---|---|---|---|
| P1 | Schisandrin a | 2.17 | 433.2210 | C24H32O7 | 30, 60, 120 |
| P2 | Gomisin D | 2.37 | 553.2228 | C28H34O10 | 30, 60, 120, 360, 480 |
| P3 | Schisandrol B a | 2.55 | 439.1723 | C23H28O7 | 30, 60, 120 |
| P4 | Benzoylgomisin H | 3.12 | 523.2281 | C30H34O8 | 60, 120, 360 |
| P5 | Angeloylgomisin Q | 3.51 | 553.2417 | C29H38O9 | 30, 60, 120 |
| P6 | Gomisin G | 4.08 | 537.2090 | C30H32O9 | 30, 60, 120, 360, 480 |
| P7 | Gomisin K | 4.19 | 403.2094 | C23H30O6 | 30, 60, 120, 360 |
| P8 | Gomisin E | 4.54 | 515.2264 | C28H34O9 | 30, 60, 120, 360, 480 |
| P9 | Deoxyschizandrin a | 6.84 | 417.2258 | C24H32O6 | 30, 60, 120, 360, 480 |
| P10 | Schisandrin B a | 10.21 | 401.1948 | C23H28O6 | 30, 60, 120, 360, 480 |
| Name | Metabolic Pathways | RT (min) | Measured Mass | Formula | Mass Error (ppm) | Time Points (min) | MS2 |
|---|---|---|---|---|---|---|---|
| Schisandrin a | 2.17 | 433.2210 | C24H32O7 | −2.4 | 30, 60, 120 | ||
| M1 | Hydroxylation+demethylation | 1.46 | 457.1823 | C23H30O8 | −2 | 30, 60, 120, 360, 480 | 399, 385, 354, 367 |
| M2 | 2 × Demethylation | 1.48 | 427.1695 | C22H28O7 | 1.48 | 60, 360 | 387, 385, 354 |
| M3 | Hydroxylation | 1.7 | 449.2158 | C24H32O8 | −2.6 | 30, 60, 120, 360, 480 | 413, 359,383 |
| M4 | Demethylation | 1.74 | 419.2054 | C23H30O7 | −2.4 | 30, 60, 120, 360 | 401, 373, 370, 359 |
| M5 | Phosphorylation | 1.99 | 513.1912 | C24H33O10P | 5.5 | 360, 480 | 415, 384, 385,373 |
| M6 | 2 × Demethylation+dehydration | 5.2 | 387.1786 | C22H26O6 | −3.9 | 30, 60, 120, 360, 480 | 385, 354, 338, 323 |
| Schisandrol B a | 2.54 | 439.1737 | C23H28O7 | 2.2 | 30, 60, 120 | ||
| M7 | 2 × Demethylation | 1.46 | 389.1594 | C21H24O7 | 0.2 | 30, 60, 120, 360, 480 | 355, 322, 294 |
| M8 | Reduction+demethylation | 1.48 | 427.1695 | C22H28O7 | −7.5 | 60, 360 | 387, 331, 345 |
| M9 (Schisandrin) | Reduction+methylation | 2.17 | 433.2210 | C24H32O7 | −2.4 | 30, 60, 120 | 415, 400, 385, 384, 373, 369, 359, 354, 353, 338, 322 |
| M10 | CH2O (cleavage)+dehydration | 2.54 | 369.1673 | C22H24O5 | −6.4 | 30, 60, 120 | 353, 337, 264 |
| M11 | Reduction+dehydration | 3.12 | 401.1923 | C23H28O6 | −9 | 360 | 370, 345, 359, 386 |
| M12 | Reduction + dehydration + demethylation | 5.2 | 387.1786 | C22H26O6 | −4.1 | 30, 60, 120, 360, 480 | 331, 345 |
| Angeloylgomisin Q | 3.51 | 553.2417 | C29H38O9 | 1.7 | 30, 60, 120 | ||
| M9 (Schisandrin) | C5H6O2 (cleavage) | 2.17 | 433.2210 | C24H32O7 | −2.4 | 30, 60, 120 | 415, 400, 385, 384 |
| M13 | Demethylation | 2.27 | 539.2239 | C28H36O9 | −2.2 | 30, 60, 120 | 417, 399, 389, 387, 369 |
| Gomisin G | 4.08 | 537.2090 | C30H32O9 | −5.4 | 30, 60, 120, 360, 480 | ||
| M14 | C7H4O2(cleavage) + 2 × demethylation | 1.46 | 389.1594 | C21H24O7 | 0.2 | 30, 60, 120, 360, 480 | 371, 356, 340 |
| M15 | Demethylation | 3.62 | 523.1932 | C29H30O9 | −5.7 | 30, 120, 360 | 401, 383, 369, 357, |
| Gomisin D | 2.37 | 531.2228 | C28H34O10 | 0.7 | 30, 60, 120, 360, 480 | ||
| M16 | C6H8O3(cleavage) + reduction | 1.48 | 427.1695 | C22H28O7 | −7.6 | 60, 360, 480 | 387, 359, 355 |
| M17 | Reduction | 1.6 | 555.2165 | C28H36O10 | −6.5 | 30, 60, 120, 360, 480 | 485, 383, 352, 351, 341 |
| M18 | C6H8O3(cleavage) | 1.63 | 425.1545 | C22H26O7 | −5.9 | 360, 480 | 385, 355, 353 |
| M19 | Demethylation | 1.81 | 539.1884 | C27H32O10 | −0.5 | 360, 480 | 387, 368, 357 |
| M20 | C6H8O2(cleavage) | 2.3 | 419.1666 | C22H26O8 | −8.3 | 30, 360 | 401, 383, 371, 351 |
| M21 | C6H8O3(cleavage) + dehydration | 2.35 | 385.1629 | C22H24O6 | −4.3 | 30, 60, 120, 360, 480 | 355, 353 |
| M22 | C6H8O2(cleavage) + reduction | 2.36 | 443.1699 | C22H28O8 | 5.1 | 60, 120, 360, 480 | 403, 385, 357, 351 |
| M23 | Phosphorylation | 3.62 | 611.1859 | C28H35O13P | −4.8 | 60, 120, 360, 480 | 383, 371, 351 |
| Gomisin E | 4.54 | 515.2264 | C28H34O9 | −2.2 | 30, 60, 120, 360, 480 | ||
| M24 | C6H8O2(cleavage) | 1.73 | 403.1761 | C22H26O7 | 2.5 | 120 | 385, 355, 354, 353, 343 |
| M25 | Decarboxylation | 2.21 | 471.2388 | C27H34O7 | 2.2 | 360, 480 | 385, 355, 354, 353, 343 |
| M26 | Reduction | 2.27 | 539.2239 | C28H36O9 | −2.4 | 30, 60, 120 | 469, 355, 354, 353, 343, 329 |
| M27 | Hydration | 4.77 | 555.2166 | C28H36O10 | −6.4 | 60, 360 | 385, 355, 354, 343 |
| Benzoylgomisin H | 3.12 | 523.2281 | C30H34O8 | −8.7 | 60, 120, 360 | ||
| M28 | C7H4O(cleavage) | 1.73 | 419.2061 | C23H30O7 | −0.6 | 30, 60, 120 | 401, 385, 316 |
| Gomisin K | 4.19 | 403.2094 | C23H30O6 | −5.3 | 30, 60, 120, 360 | ||
| M29 | Hydroxylation + demethylation | 1.48 | 427.1695 | C22H28O7 | −7.3 | 60 | 387, 372, 355, 333, 302 |
| M30 | Demethylation | 1.72 | 389.1934 | C22H28O6 | −6.3 | 30, 60 | 374, 357, 333, 302 |
| M31 | Hydroxylation | 1.73 | 419.2061 | C23H30O7 | −0.8 | 30, 60, 120, 360 | 401, 386, 369, 333, 302 |
| M32 | 2×Desaturation + acetyl cysteine conjugation | 1.75 | 590.2380 | C28H41NO9S | −2.6 | 30, 60, 120, 360, 480 | 389, 373, 359, 319 |
| Deoxyschizandrin a | 6.84 | 417.2258 | C24H32O6 | −3.2 | 30, 60, 120, 360, 480 | ||
| M9 (Schisandrin) | Hydroxylation | 2.18 | 433.2210 | C24H32O7 | −2.4 | 30, 60, 120 | 415, 400, 385, 384, 373, 369, 359, 354, 353, 338, 322 |
| M28 | Demethylation + hydroxylation | 1.73 | 419.2061 | C23H30O7 | −0.6 | 30, 60, 120 | 401, 370, 369, 337 |
| M33 | Demethylation | 4.65 | 403.2093 | C23H30O6 | −5.3 | 30, 60, 120, 360, 480 | 388, 385, 372, 371, 370, 339 |
| M34 | 2 × Hydroxylation | 1.7 | 449.2154 | C24H32O8 | −3.5 | 30, 60, 120, 360, 480 | 431, 413, 398, 383, 343, 316 |
| M35 | 2 × Desaturation | 3.48 | 413.1944 | C24H28O6 | −3.6 | 60, 120, 360, 480 | 398, 383, 382, 366, 347, 316 |
| Schisandrin B a | 10.21 | 401.1948 | C23H28O6 | −2.7 | 30, 60, 120, 360, 480 | ||
| M36 | Reduction + phosphorylation | 1.68 | 483.1799 | C23H31O9P | 4.1 | 30, 60, 120, 360 | 387 |
| M37 | Demethylation + phosphorylation | 1.81 | 467.1488 | C22H27O9P | 4.8 | 60 | 370, 371,300 |
| M38 (Schisandrol B) | Hydroxylation | 2.54 | 439.1737 | C23H28O7 | 2.2 | 30, 60, 120 | 399, 384, 369, 368, 357, 353, 343, 341, 337, 338, 295 |
| M39 (Deoxyschizandrin) | Reduction + methylation | 6.84 | 417.2258 | C24H32O6 | −3.2 | 30, 60, 120, 360, 480 | 402, 386, 370, 371, 355, 347, 332, 316 |
| Target Gene | Pathway | Relative Target | Relative Pathway | Effective Constituents |
|---|---|---|---|---|
| AChE | Cholinergic synapse | APP | amyloid precursor protein metabolism | Gomisin K; Gomisin G |
| iNOS | Arginine and proline metabolism | Neurotransmitter; Calmodulin; IL | Nitric oxide anabolism; calmodulin binding; neurotransmitter metabolism; inflammatory response | Gomisin K |
| PTGS1 | Arachidonic acid metabolism | PGs | Platelet activation; Cytochrome P450 - arranged by substrate type | Gomisin K |
| PTGS2 | Arachidonic acid metabolism | PGs | inflammatory response; Cytokine signaling in immune system | Gomisin K; Benzoylgomisin H; Schisandrol B; Gomisin D |
| GSK3β | PI3K-Akt signaling pathway; Wnt signaling pathway | Tau; DA | MAPK signaling pathway; Dopaminergic synapse; neurofibrillary tangles | Gomisin K |
| TGFB1 | MAPK signaling pathway | TNF-α | TNF signaling pathway | Schisandrin B |
| MAPK1 | MAPK signaling pathway | Glu; ACh; 5-HT | Glutamatergic synapse; Cholinergic synapse; Serotonergic synapse | Schisandrin B |
| MAPK3 | MAPK signaling pathway | Glu; ACh; 5-HT | Glutamatergic synapse; Cholinergic synapse; Serotonergic synapse | Schisandrin B |
| TBXAS1 | Cytochrome P450 - arranged by substrate type | AA | Arachidonic acid metabolism; Platelet activation | Schisandrin B |
| CYP3A4 | Drug metabolism - cytochrome P450 | Cholesterol; LA; Retinoate | Steroid hormone biosynthesis; Linoleic acid metabolism; Retinol metabolism | Schisandrin B |
| PPARG | PPAR signaling pathway | 9-CRA | Lipid metabolism; Antioxidant system | Gomisin K |
| TOP2 | Metabolism of proteins | / | Cell cycle | Gomisin K; Angeloylgomisin Q; Benzoylgomisin H; Schisandrol B; Gomisin D; Gomisin G |
| Mir34a | MicroRNAs in cancer | Cyclin-dependent kinase 6 | Cell cycle | Schisandrin B |
| CCND1 | Cyclins and cell cycle regulation | / | / | Schisandrin B |
| GSR | Glutathione metabolism | GSH | Glutathione metabolism; Antioxidant system | Schisandrin B |
| NFE2L2 | Protein processing in endoplasmic reticulum | HMOX1 | Porphyrin and chlorophyll metabolism | Schisandrin B; Deoxyschizandrin |
| HMOX1 | Porphyrin and chlorophyll metabolism | APP; IL-10; NFE2L2 | amyloid precursor protein metabolism; Protein processing in endoplasmic reticulum;Cytokine signaling in immune system | Schisandrin |
| Description | Formula | Delta Mass (Da) | Classifier |
|---|---|---|---|
| Parent | / | / | / |
| Methylation | +CH2 | 14.0157 | Phase II |
| Demethylation | –CH2 | −14.0157 | Phase I |
| Reduction | +H2 | 2.0157 | Phase I |
| Desaturation | –H2 | −2.0157 | Phase I |
| 2×Desaturation | –H4 | −4.0313 | Phase I |
| Hydroxylation | +O | 15.9949 | Phase I |
| 2×Hydroxylation | +O2 | 31.9898 | Phase I |
| 3×Hydroxylation | +O3 | 47.9847 | Phase I |
| Nitro reduction | –O2+H2 | −29.9742 | Phase I |
| Dehydration | –H2O | −18.0106 | Phase I, II |
| Hydration | +H2O | 18.0106 | Phase I |
| Dihydrodiol formation | +H2O2 | 34.0055 | Phase I |
| Decarbonylation | –-CO | −27.9949 | Phase I |
| Formylation | +CO | 27.9949 | Phase II |
| Decarboxylation | –COO | −43.9898 | Phase I |
| Phosphorylation | +HPO3 | 79.9663 | Phase II |
| Acetyl cysteine conjugation | +C5H7NO3S | 161.0147 | Phase II |
| 2 × Glucuronide conjugation | +C12H16O12 | 352.0642 | Phase II |
| Glucuronidation | +C6H8O6 | 176.0321 | Phase II |
| Mode | Compounds | Capillary Voltage (kV) | Nebulizer Gas (L/h) | Desolvation Gas (L/h) | Cone Voltage (V) | Quantitation Transition (m/z, Collision (eV)1) | Confirmation Transition (m/z, Collision (eV) 1) |
|---|---|---|---|---|---|---|---|
| ESI+ | Gly | 2.5 | 50 | 800 | 14 | 75.97 > 30.19 (8) | 75.97 > 48.14 (6) |
| Asp | 2.5 | 50 | 800 | 12 | 133.97 > 74.03 (14) | 133.97 > 88.07 (10) | |
| Glu | 2.5 | 50 | 800 | 14 | 147.97 > 84.11 (16) | 147.97 > 130.03 (8) | |
| GABA | 2.5 | 50 | 800 | 20 | 103.97 > 86.99 (10) | 103.97 > 68.03 (14) | |
| NE | 2.5 | 50 | 800 | 6 | 169.97 > 152.04 (8) | 169.97 > 107.3 (18) | |
| ACh | 2.5 | 50 | 800 | 22 | 146.03 > 87.04 (12) | 146.01 > 60.11 (10) | |
| DA | 2.5 | 50 | 800 | 12 | 153.97 > 137 (10) | 153.97 > 91.08 (22) | |
| 5-HT | 2.5 | 50 | 800 | 10 | 176.97 > 160.02 (8) | 176.97 > 132.06 (20) | |
| 9-CRA | 3 | 40 | 500 | 20 | 301.29 > 123.17 (22) | 301.29 > 161.40 (22) | |
| ESI− | AA | 2.6 | 50 | 650 | 32 | 303.35 > 259.29 (12) | 303.35 > 205.21 (16) |
| LA | 2.8 | 40 | 500 | 36 | 279.35 > 261.23 (18) | 279.35 > 59.13 (18) | |
| cholesterol sulfate | 2.6 | 50 | 650 | 62 | 465.48 > 97.03 (36) | 465.48 > 80.00 (80) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Liu, Y.; Pi, Z.; Li, S.; Hu, M.; He, Y.; Yue, K.; Liu, T.; Liu, Z.; Song, F.; et al. Systematically Characterize the Anti-Alzheimer’s Disease Mechanism of Lignans from S. chinensis Based on In-Vivo Ingredient Analysis and Target-Network Pharmacology Strategy by UHPLC–Q-TOF-MS. Molecules 2019, 24, 1203. https://doi.org/10.3390/molecules24071203
Wei M, Liu Y, Pi Z, Li S, Hu M, He Y, Yue K, Liu T, Liu Z, Song F, et al. Systematically Characterize the Anti-Alzheimer’s Disease Mechanism of Lignans from S. chinensis Based on In-Vivo Ingredient Analysis and Target-Network Pharmacology Strategy by UHPLC–Q-TOF-MS. Molecules. 2019; 24(7):1203. https://doi.org/10.3390/molecules24071203
Chicago/Turabian StyleWei, Mengying, Yuanyuan Liu, Zifeng Pi, Shizhe Li, Mingxin Hu, Yang He, Kexin Yue, Tianshu Liu, Zhiqiang Liu, Fengrui Song, and et al. 2019. "Systematically Characterize the Anti-Alzheimer’s Disease Mechanism of Lignans from S. chinensis Based on In-Vivo Ingredient Analysis and Target-Network Pharmacology Strategy by UHPLC–Q-TOF-MS" Molecules 24, no. 7: 1203. https://doi.org/10.3390/molecules24071203
APA StyleWei, M., Liu, Y., Pi, Z., Li, S., Hu, M., He, Y., Yue, K., Liu, T., Liu, Z., Song, F., & Liu, Z. (2019). Systematically Characterize the Anti-Alzheimer’s Disease Mechanism of Lignans from S. chinensis Based on In-Vivo Ingredient Analysis and Target-Network Pharmacology Strategy by UHPLC–Q-TOF-MS. Molecules, 24(7), 1203. https://doi.org/10.3390/molecules24071203