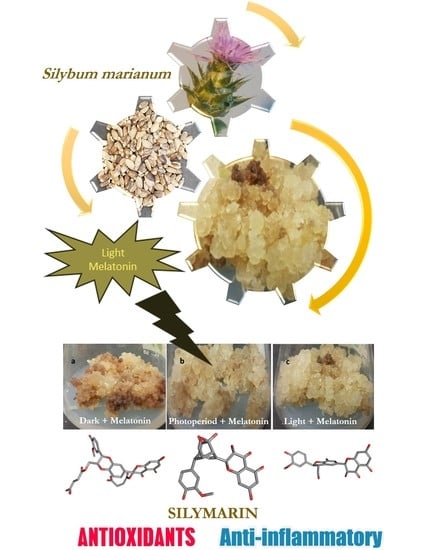
Interactive Effects of Light and Melatonin on Biosynthesis of Silymarin and Anti-Inflammatory Potential in Callus Cultures of Silybum marianum (L.) Gaertn.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Interactive Effect of Light and Melatonin on Biomass Accumulation
2.2. Interactive Effect of Light and Melatonin on Accumulation of Secondary Metabolites
2.3. Effect of Light and Melatonin on Antioxidant Activities
2.4. Effect of Light and Melatonin on Anti-Inflammatory Potential of S. Marianum Callus Cultures
2.5. Effect of Light and Melatonin on Silymarin
3. Materials and Methods
3.1. Chemicals
3.2. Seed Germination and Explant Collection
3.3. Callus Culture Establishment
3.4. Melatonin and Light Treatment
3.5. Total Flavonoid and Phenolic Contents
3.6. Estimation of Antioxidant Activity
3.6.1. DPPH Activity (%)
3.6.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6.3. Antioxidant ABTS Assay
3.7. Anti-Inflammatory Activities
3.7.1. Inhibitory Activity Against COX-1 and COX-2
3.7.2. Inhibitory Activity Against 15-LOX
3.7.3. Inhibitory Activity Against Secretory Phospholipase A2 (sPLA2)
3.8. High-Performance Liquid Chromatography Electrospray Ionization Mass Spectrometry (HPLC-ESI-MS) Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Abbasi, B.H.; Ahmed, N.; Ali, H. Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind. Crops Prod. 2013, 46, 105–110. [Google Scholar] [CrossRef]
- Soto, C.; Pérez, J.; García, V.; Uría, E.; Vadillo, M.; Raya, L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine 2010, 17, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Al-Anati, L.; Essid, E.; Reinehr, R.; Petzinger, E. Silibinin protects OTA-mediated TNF-α release from perfused rat livers and isolated rat Kupffer cells. Mol. Nutr. Food Res. 2009, 53, 460–466. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.-Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, M.; Guo, B.; Bokhari, S.A.; Khan, M.A. Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tissue Organ. Cult. (PCTOC) 2011, 105, 337–344. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.K.; Wahi, A.; Brijesh, K.; Bhandari, A.; Prasad, N. Milk thistle (Silybum marianum): A review. IJPRD 2011, 3, 1–10. [Google Scholar]
- Polyak, S.J.; Ferenci, P.; Pawlotsky, J.M. Hepatoprotective and antiviral functions of silymarin components in hepatitis C virus infection. Hepatology 2013, 57, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Can, V.; Abouelnour, A.; Locke, I.; Bligh, S.; Getting, S. The effect of silymarin on chondrocytes. Biochem. Pharmacol. 2017, 139, 137. [Google Scholar] [CrossRef]
- Qin, N.-b.; Jia, C.-c.; Xu, J.; Li, D.-h.; Xu, F.-x.; Bai, J.; Li, Z.-l.; Hua, H.-m. New amides from seeds of Silybum marianum with potential antioxidant and antidiabetic activities. Fitoterapia 2017, 119, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, M.; Moini, A.; Shakiba, Y.; Mashkorinia, A.; Dehghani, M.; Asar, S.; Kiani, A. Silymarin (Livergol®) decreases disease activity score in patients with rheumatoid arthritis: A non-randomized single-Arm clinical trial. Iran J. Allergy Asthma Immunol. 2017, 16, 99–106. [Google Scholar]
- Grant, J.E.; Odlaug, B.L. Silymarin treatment of obsessive-compulsive spectrum disorders. J. Clin. Psychopharmacol. 2015, 35, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Boccuto, L.; Abenavoli, L. Silymarin: An option to treat non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8437. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, B.; Gharagozloo, M.; Esmaeil, N.; Maracy, M.R.; Hoorfar, H.; Jalaeikar, M. A randomized double-blind, placebo-controlled study of therapeutic effects of silymarin in β-thalassemia major patients receiving desferrioxamine. Eur. J. Haematol. 2013, 90, 202–209. [Google Scholar] [CrossRef]
- Mohale, D.; Tripathi, A.; Wahane, J.; Chandewar, A. A Pharmacological Review on Cyclooxygenase Enzyme. Ind. J. Pharm. Pharmacol. 2014, 1, 46–58. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phytother Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted lignan profiling and anti-inflammatory properties of Schisandra rubriflora and Schisandra chinensis extracts. Molecules 2018, 23, 3103. [Google Scholar] [CrossRef]
- Borges, A.; Casoti, R.; e Silva, M.L.A.; da Cunha, N.L.; da Rocha Pissurno, A.P.; Kawano, D.F.; da Silva de Laurentiz, R. COX Inhibition Profiles and Molecular Docking Studies of the Lignan Hinokinin and Some Synthetic Derivatives. Mol. Inform. 2018, 37, 1800037. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Hughes, K.W. In vitro ecology: Exogenous factors affecting growth and morphogenesis in plant culture systems. Environ. Exp. Bot. 1981, 21, 281–288. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Cao, J.; Murch, S.J.; O’Brien, R.; Saxena, P.K. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2006, 1134, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal. Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Kolář, J.; Macháčková, I. Melatonin in higher plants: Occurrence and possible functions. J. Pineal. Res. 2005, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Tan, D.-X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious-and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal. Res. 2007, 42, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Saxena, P.K. Melatonin: A potential regulator of plant growth and development? In Vitro Cell Dev. Biol. Plant. 2002, 38, 531–536. [Google Scholar] [CrossRef]
- Kolář, J.; Johnson, C.H.; Macháčková, I. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol. Plant 2003, 118, 605–612. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal. Res. 2012, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Kolář, J.; Witters, E.; van Dongen, W.; van Onckelen, H.; Macháčková, I. Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J. Plant Physiol. 2001, 158, 1491–1493. [Google Scholar] [CrossRef]
- Fankhauser, C.; Chory, J. Light control of plant development. Annu. Rev. Cell Dev. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2014, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2014, 66, 647–656. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Exogenous melatonin trigger biomass accumulation and production of stress enzymes during callogenesis in medicinally important Prunella vulgaris L. (Selfheal). Physiol. Mol. Biol. Plants. 2018, 24, 1307–1315. [Google Scholar] [CrossRef]
- Khan, T.; Ullah, M.A.; Garros, L.; Hano, C.; Abbasi, B.H. Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J. Photochem. Photobiol. B 2018. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Abbasi, B.H.; Khan, T. Interactive effects of melatonin and light on growth parameters and biochemical markers in adventitious roots of Withania somnifera L. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 405–412. [Google Scholar] [CrossRef]
- Tan, J.; Bednarek, P.; Liu, J.; Schneider, B.; Svatoš, A.; Hahlbrock, K. Universally occurring phenylpropanoid and species-specific indolic metabolites in infected and uninfected Arabidopsis thaliana roots and leaves. Phytochemistry 2004, 65, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Khatun, S.; Hahn, E.-J.; Paek, K.-Y. Enhancement of phenylpropanoid enzymes and lignin in Phalaenopsis orchid and their influence on plant acclimatisation at different levels of photosynthetic photon flux. Plant Growth Regul. 2006, 49, 137–146. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I.; Koularmani, A. Effect of melatonin, salicylic acid and gibberellic acid on leaf essential oil and other secondary metabolites of bitter orange young seedlings. J. Essent. Oil Res. 2015, 27, 487–496. [Google Scholar] [CrossRef]
- Ellis, R.; Roberts, E. Towards a rational basis for testing seed quality. Proc.-Easter Sch. Agric. Sci. Univ. Nottm. 1978, 605–635. [Google Scholar]
- Senger, H. Blue Light Responses: Phenomena and Occurrence in Plants and Microorganisms; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Zhong, J.J.; Seki, T.; Kinoshita, S.i.; Yoshida, T. Effect of light irradiation on anthocyanin production by suspended culture of Perilla frutescens. Biotechnol. Bioeng. 1991, 38, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.G.; Zhang, Y.; Seeram, N.P.; Cameron, A.C.; Nair, M.G. Relationship of light quantity and anthocyanin production in Pennisetum setaceum cvs. Rubrum and Red Riding Hood. J. Agric. Food. Chem. 2004, 52, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Yu, T.-W.; Anderson, D. Reactive oxygen species-induced DNA damage and its modification: A chemical investigation. Mutat. Res. Fund. Mol. M 1997, 379, 201–210. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ashry, N.A.; Mohamed, H.I. Impact of secondary metabolites and related enzymes in flax resistance and or susceptibility to powdery mildew. World J. Agric. Sci 2011, 7, 78–85. [Google Scholar]
- Samuolienė, G.; Brazaitytė, A.; Urbonavičiūtė, A.; Šabajevienė, G.; Duchovskis, P. The effect of red and blue light component on the growth and development of frigo strawberries. Zemdirbyste Agric. 2010, 97, 99–104. [Google Scholar]
- Basaga, H.; Poli, G.; Tekkaya, C.; Aras, I. Free radical scavenging and antioxidative properties of ‘silibin’complexes on microsomal lipid peroxidation. Cell Biochem. Func. 1997, 15, 27–33. [Google Scholar] [CrossRef]
- Zahir, A.; Abbasi, B.H.; Adil, M.; Anjum, S.; Zia, M. Synergistic effects of drought stress and photoperiods on phenology and secondary metabolism of Silybum marianum. Appl. Biochem. Biotechnol. 2014, 174, 693–707. [Google Scholar] [CrossRef]
- Lettéron, P.; Labbe, G.; Degott, C.; Berson, A.; Fromenty, B.; Delaforge, M.; Larrey, D.; Pessayre, D. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice: Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem. Pharmacol. 1990, 39, 2027–2034. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food. Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.; Sing, S.; Bani, S.; Sharma, N.; Malhotra, S.; Gupta, B.; Banerjee, S.; Handa, S. Anti-inflammatory and anti-arthritic activities of silymarin acting through inhibition of 5-lipoxygenase. Phytomedicine 2000, 7, 21–24. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Amini, R.; Yazdanparast, R.; Ghaffari, S.H. Anti-apoptotic and anti-inflammatory effects of Silybum marianum in treatment of experimental steatohepatitis. Exp. Toxicol. Pathol. 2011, 63, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Shaker, E.; Mahmoud, H.; Mnaa, S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010, 48, 803–806. [Google Scholar] [CrossRef]
- Pradhan, S.; Girish, C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006, 124, 491–504. [Google Scholar]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.; Ling, A.P.K.; Hussein, S. Callus induction of Ocimum sanctum and estimation of its total flavonoids content. Asian J. Agric. Sci. 2009, 1, 55–61. [Google Scholar]
- Hackett, E.; Twedt, D.; Gustafson, D. Milk thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J. Vet. Intern. Med. 2013, 27, 10–16. [Google Scholar] [CrossRef]
- Surai, P. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Althagafy, H.S.; Meza-Aviña, M.E.; Oberlies, N.H.; Croatt, M.P. Mechanistic study of the biomimetic synthesis of flavonolignan diastereoisomers in milk thistle. J. Org. Chem. 2013, 78, 7594–7600. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.-n.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Zahir, A.; Hano, C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J. Photochem. Photobiol. B 2018. [Google Scholar] [CrossRef]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J. Photochem. Photobiol. B 2018, 184, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Merlini, L.; Zanarotti, A.; Pelter, A.; Rochefort, M.P.; Hänsel, R. Benzodioxans by oxidative phenol coupling. Synthesis of silybin. J. Chem. Soc. Perkin Trans. 1 1980, 775–778. [Google Scholar] [CrossRef]
- Poppe, L.; Petersen, M. Variation in the flavonolignan composition of fruits from different Silybum marianum chemotypes and suspension cultures derived therefrom. Phytochemistry 2016, 131, 68–75. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Rashid, M.; Mahmood, T.; Fatima, N. Efficient regeneration and antioxidant potential in regenerated tissues of Piper nigrum L. Plant Cell Tissue Organ. Cult. 2010, 102, 129–134. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, M.A.; Mahmood, T.; Ahmad, M.; Chaudhary, M.F.; Khan, M.A. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant. Cell Tissue Organ. Cult. 2010, 101, 371–376. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Drouet, S.; Abbasi, B.; Falguières, A.; Ahmad, W.; Ferroud, C.; Doussot, J.; Vanier, J.; Lainé, E.; Hano, C. Single laboratory validation of a quantitative core shell-based LC separation for the evaluation of silymarin variability and associated antioxidant activity of pakistani ecotypes of milk thistle (Silybum marianum L.). Molecules 2018, 23, 904. [Google Scholar] [CrossRef]
- Gargari, B.P.; Mobasseri, M.; Valizadeh, H.; Asghari-Jafarabadi, M. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: A randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015, 22, 290–296. [Google Scholar]
- Hussain, S.A.; Jassim, N.A.; Numan, I.T.; Al-Khalifa, I.I.; Abdullah, T.A. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. A comparative study with piroxicam and meloxicam. Saudi Med. J. 2009, 30, 98–103. [Google Scholar] [PubMed]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Plant materials and chemical standards used in the present study are available upon request to corresponding authors. |







| Treatment | Dark (24 h) | Photoperiod (16 h/8 h) | Continuous Light (24 h) | |||
|---|---|---|---|---|---|---|
| Melatonin (mg/L) | FW (g/L) | DW (g/L) | FW (g/L) | DW (g/L) | FW (g/L) | DW (g/L) |
| Control | 170.47 ± 3.83 d | 10.74 ± 0.38 bc | 188.9 ± 5.38 cd | 11.27 ± 0.64 bc | 215.4 ± 4.07 c | 12.56 ± 0.50 b |
| 0.1 | 210.888 ± 4.09 c | 12.36 ± 0.25 b | 149.8 ± 4.30 de | 12.78 ± 0.53 b | 252.6 ± 6.42 b | 13.93 ± 0.57 ab |
| 0.5 | 159.9 ± 5.20 de | 13.11 ± 1.37 ab | 177.14 ± 4.86 d | 14.37 ± 0.13 ab | 266.7 ± 7.6 ab | 14.29 ± 0.83 ab |
| 1.0 | 135.78 ± 7.78 e | 10.79 ± 0.90 bc | 205.88 ± 3.42 c | 13.87 ± 0.92 ab | 299.3 ± 6.57 a | 15.96 ± 0.68 a |
| 2.5 | 131.58 ± 6.03 e | 10.66 ± 0.49 bc | 175.01 ± 4.46 d | 10.39 ± 0.50 bc | 204.3 ± 9.72 c | 12.57 ± 0.37 b |
| 5.0 | 101.44 ± 4.75 ef | 8.26 ± 0.50 c | 165.94 ± 5.92 d | 12.33 ± 0.35 b | 215.5 ± 8.28 c | 13.10 ± 1.01 ab |
| 10.0 | 93.46 ± 3.85 f | 7.54 ± 0.46 cd | 152.6 ± 5.73 de | 11.65 ± 0.42 b | 260.5 ± 6.8 ab | 14.73 ± 1.34 a |
| Light Regime | Melatonin (mg/L) | 15-LOX (% Inh) | COX-1 (% Inh) | sPLA2 (% Inh) | COX-2 (% Inh) |
|---|---|---|---|---|---|
| Dark | Control | 18.56 ± 1.05 c | 20.55 ± 1.08 cd | 19.83 ± 1.45 bc | 13.37 ± 1.34 bc |
| 0.1 | 20.90 ± 1.43 c | 21.20 ± 1.30 cd | 22.27 ± 0.95 bc | 15.93 ± 2.01 bc | |
| 0.5 | 30.99 ± 0.99 b | 28.60 ± 1.54 b | 28.96 ± 1.03 ab | 22.24 ± 1.34 ab | |
| 1.0 | 28.74 ± 2.56 b | 26.94 ± 0.83 bc | 27.37 ± 1.30 ab | 20.78 ± 0.94 b | |
| 2.5 | 26.41 ± 1.98 bc | 25.51 ± 1.54 bc | 25.08 ± 1.28 b | 19.05 ± 1.56 b | |
| 5.0 | 21.38 ± 2.03 c | 21.47 ± 1.48 cd | 22.70 ± 1.84 bc | 16.25 ± 2.01 bc | |
| 10.0 | 20.99 ± 3.51 c | 21.07 ± 1.56 cd | 22.62 ± 1.26 bc | 16.05 ± 1.67 bc | |
| Photoperiod | Control | 21.87 ± 2.50 c | 21.62 ± 1.62 cd | 23.42 ± 1.49 bc | 16.68 ± 0.93 bc |
| 0.1 | 30.13 ± 1.87 b | 27.90 ± 1. 38 b | 28.80 ± 0.84 ab | 21.89 ± 1.45 ab | |
| 0.5 | 22.59 ± 1.14 bc | 22.48 ± 2.05 c | 23.23 ± 2.03 b | 16.92 ± 1.04 bc | |
| 1.0 | 24.07 ± 1.41 bc | 23.47 ± 1.56 c | 24.35 ± 1.82 b | 17.88 ± 0.72 bc | |
| 2.5 | 18.72 ± 1.78 c | 19.51 ± 0.83 cd | 21.02 ± 0.59 bc | 14.63 ± 1.94 bc | |
| 5.0 | 28.45 ± 1.05 b | 26.64 ± 1.55 bc | 27.58 ± 1.47 ab | 20.77 ± 2.04 b | |
| 10.0 | 26.36 ± 1.32 bc | 24.66 ± 1.83 c | 22.98 ± 2.44 bc | 18.66 ± 1.43 b | |
| Continuous Light | Control | 28.37 ± 1.69 b | 22.66 ± 0.80 c | 21.79 ± 1.28 bc | 16.23 ± 1.93 bc |
| 0.1 | 23.65 ± 0.97 bc | 23.09 ± 0.92 c | 24.25 ± 1.66 b | 17.68 ± 1.03 bc | |
| 0.5 | 31.49 ± 1.23 b | 28.97 ± 1.56 b | 29.35 ± 1.22 ab | 22.59 ± 1.05 ab | |
| 1.0 | 42.33 ± 1.59 a | 37.15 ± 1.29 a | 35.70 ± 0.99 a | 29.03 ± 0.97 a | |
| 2.5 | 29.95 ± 1.86 b | 27.80 ± 1.45 b | 28.35 ± 2.76 ab | 21.62 ± 1.46 ab | |
| 5.0 | 30.87 ± 1.23 b | 28.52 ± 1.33 b | 28.76 ± 1.55 ab | 22.11 ± 1.35 ab | |
| 10.0 | 30.82 ± 1.50 b | 28.45 ± 1.20 b | 29.00 ± 1.39 ab | 22.20 ± 1.04 ab |
| Light Regimes | Melatonin (mg/L) | Silymarin Compounds (mg/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Silybin A | Silybin B | Isosilybin A | Isosilybin B | Silychristin | Isosilychristin | Silydianin | Taxifolin | ||
| Dark | Control | 0.62 ± 0.05 cd | 3.34 ± 0.04 e | 0.17 ± 0.002 b | 0.15 ± 0.03 bc | 0.58 ± 0.06 c | 0.28 ± 0.02 bc | 0.60 ± 0.08 bc | 0.05 ± 0.009 b |
| 0.1 | 0.67 ± 0.002 cd | 3.67 ± 0.06 de | 0.19 ± 0.04 ab | 0.15 ± 0.02 bc | 0.60 ± 0.03 c | 0.29 ± 0.09 bc | 0.61 ± 0.064 b | 0.06 ± 0.005 b | |
| 0.5 | 1.06 ± 0.045 b | 5.59 ± 0.076 bc | 0.22 ± 0.055 a | 0.22 ± 0.054 ab | 0.82 ± 0.028 b | 0.38 ± 0.059 ab | 0.68 ± 0.055 ab | 0.13 ± 0.003 a | |
| 1.0 | 0.97 ± 0.037 bc | 5.13 ± 0.056 c | 0.21 ± 0.029 ab | 0.21 ± 0.053 b | 0.77 ± 0.048 bc | 0.36 ± 0.040 b | 0.66 ± 0.087 b | 0.08 ± 0.006 ab | |
| 2.5 | 0.82 ± 0.039 c | 4.37 ± 0.042 d | 0.21 ± 0.019 ab | 0.19 ± 0.048 b | 0.73 ± 0.098 bc | 0.33 ± 0.076 ab | 0.67 ± 0.067 ab | 0.08 ± 0.004 ab | |
| 5.0 | 0.70 ± 0.048 cd | 3.81 ± 0.039 de | 0.19 ± 0.074 ab | 0.16 ± 0.047 bc | 0.60 ± 0.038 c | 0.29 ± 0.033 bc | 0.61 ± 0.062 b | 0.06 ± 0.007 b | |
| 10.0 | 0.69 ± 0.044 cd | 3.82 ± 0.027 de | 0.19 ± 0.06 ab | 0.15 ± 0.051 bc | 0.59 ± 0.064 c | 0.29 ± 0.009 bc | 0.60 ± 0.094 bc | 0.06 ± 0.008 b | |
| Photoperiod | Control | 0.74 ± 0.04 c | 4.08 ± 0.03 d | 0.19 ± 0.05 ab | 0.16 ± 0.02 bc | 0.60 ± 0.07 c | 0.30 ± 0.04 bc | 0.59 ± 0.09 bc | 0.06 ± 0.004 b |
| 0.1 | 1.05 ± 0.09 b | 5.59 ± 0.89 bc | 0.21 ± 0.04 ab | 0.21 ± 0.04 b | 0.79 ± 0.05 b | 0.37 ± 0.06 ab | 0.66 ± 0.01 b | 0.09 ± 0.002 ab | |
| 0.5 | 0.72 ± 0.06 c | 3.93 ± 0.74 de | 0.21 ± 0.05 ab | 0.16 ± 0.06 bc | 0.64 ± 0.09 c | 0.30 ± 0.08 bc | 0.63 ± 0.05 b | 0.06 ± 0.008 b | |
| 1.0 | 0.79 ± 0.048 c | 4.27 ± 0.55 d | 0.20 ± 0.07 ab | 0.17 ± 0.08 bc | 0.66 ± 0.03 c | 0.31 ± 0.07 b | 0.63 ± 0.03 b | 0.07 ± 0.006 b | |
| 2.5 | 0.60 ± 0.07 cd | 3.34 ± 0.48 e | 0.18 ± 0.095 b | 0.14 ± 0.03 bc | 0.54 ± 0.02 cd | 0.27 ± 0.03 bc | 0.59 ± 0.08 bc | 0.05 ± 0.005 b | |
| 5.0 | 0.98 ± 0.039 bc | 5.23 ± 0.95 c | 0.19 ± 0.004 ab | 0.20 ± 0.05 b | 0.75 ± 0.06 bc | 0.36 ± 0.05 b | 0.65 ± 0.09 b | 0.08 ± 0.007 ab | |
| 10.0 | 0.81 ± 0.055 c | 4.34 ± 0.33 d | 0.20 ± 0.056 ab | 0.18 ± 0.07 b | 0.67 ± 0.04 c | 0.32 ± 0.01 b | 0.63 ± 0.05 b | 0.07 ± 0.003 b | |
| Continuous Light | Control | 0.80 ± 0.05 c | 4.32 ± 0.98 d | 0.20 ± 0.03 ab | 0.17 ± 0.01 bc | 0.66 ± 0.02 c | 0.31 ± 0.07 b | 0.63 ± 0.04 b | 0.051 ± 0.005 b |
| 0.1 | 0.79 ± 0.03 c | 4.27 ± 0.07 d | 0.20 ± 0.044 ab | 0.17 ± 0.03 bc | 0.65 ± 0.06 c | 0.31 ± 0.09 b | 0.62 ± 0.08 b | 0.07 ± 0.007 b | |
| 0.5 | 1.08 ± 0.07 b | 5.71 ± 0.05 bc | 0.22 ± 0.096 a | 0.22 ± 0.07 b | 0.83 ± 0.05 b | 0.38 ± 0.03 ab | 0.68 ± 0.06 ab | 0.09 ± 0.009 ab | |
| 1.0 | 1.45 ± 0.05a | 7.43 ± 0.06 a | 0.24 ± 0.055 a | 0.30 ± 0.05 a | 1.08 ± 0.08 a | 0.47 ± 0.04 a | 0.77 ± 0.01 b | 0.13 ± 0.005 a | |
| 2.5 | 1.03 ± 0.08 b | 5.42 ± 0.04 c | 0.21 ± 0.039 ab | 0.21 ± 0.08 b | 0.79 ± 0.03 b | 0.37 ± 0.06 ab | 0.66 ± 0.05 b | 0.09 ± 0.008 ab | |
| 5.0 | 1.05 ± 0.06 b | 5.52 ± 0.098 c | 0.22 ± 0.084 a | 0.22 ± 0.04 b | 0.82 ± 0.09 b | 0.37 ± 0.032 ab | 0.68 ± 0.03 ab | 0.09 ± 0.004 ab | |
| 10.0 | 1.06 ± 0.04 b | 5.62 ± 0.05 bc | 0.22 ± 0.044 a | 0.22 ± 0.02 b | 0.81 ± 0.04 b | 0.38 ± 0.085 ab | 0.67 ± 0.07 ab | 0.08 ± 0.003 ab | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.; Ullah, M.A.; Drouet, S.; Younas, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Interactive Effects of Light and Melatonin on Biosynthesis of Silymarin and Anti-Inflammatory Potential in Callus Cultures of Silybum marianum (L.) Gaertn. Molecules 2019, 24, 1207. https://doi.org/10.3390/molecules24071207
Shah M, Ullah MA, Drouet S, Younas M, Tungmunnithum D, Giglioli-Guivarc’h N, Hano C, Abbasi BH. Interactive Effects of Light and Melatonin on Biosynthesis of Silymarin and Anti-Inflammatory Potential in Callus Cultures of Silybum marianum (L.) Gaertn. Molecules. 2019; 24(7):1207. https://doi.org/10.3390/molecules24071207
Chicago/Turabian StyleShah, Muzamil, Muhammad Asad Ullah, Samantha Drouet, Muhammad Younas, Duangjai Tungmunnithum, Nathalie Giglioli-Guivarc’h, Christophe Hano, and Bilal Haider Abbasi. 2019. "Interactive Effects of Light and Melatonin on Biosynthesis of Silymarin and Anti-Inflammatory Potential in Callus Cultures of Silybum marianum (L.) Gaertn." Molecules 24, no. 7: 1207. https://doi.org/10.3390/molecules24071207
APA StyleShah, M., Ullah, M. A., Drouet, S., Younas, M., Tungmunnithum, D., Giglioli-Guivarc’h, N., Hano, C., & Abbasi, B. H. (2019). Interactive Effects of Light and Melatonin on Biosynthesis of Silymarin and Anti-Inflammatory Potential in Callus Cultures of Silybum marianum (L.) Gaertn. Molecules, 24(7), 1207. https://doi.org/10.3390/molecules24071207