Effect of Manganese Chloride and of Cotreatment with Cadmium Chloride on the In Vitro Proliferative, Motile, and Invasive Behavior of MDA-MB231 Breast Cancer Cells
Abstract
:1. Introduction
2. Results
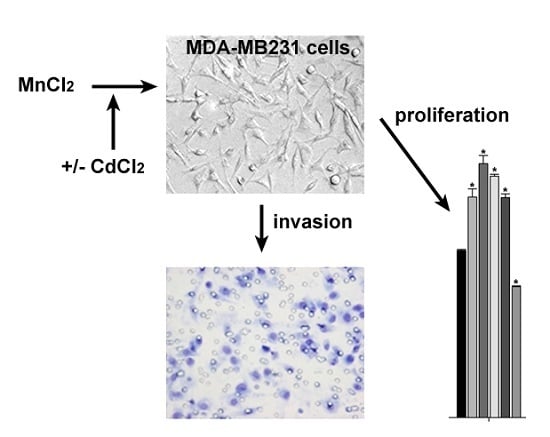
2.1. Influence of MnCl2 on Cell Proliferative and Invasive Behavior In Vitro
2.2. Effect of Coexposure to MnCl2 and CdCl2 on Cell Proliferative and Invasive Behavior In Vitro
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Proliferation Assay
4.3. In Vitro Chemotaxis and Chemoinvasion Assays
4.4. Statistics
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, Germany, 2013; pp. 199–228. [Google Scholar]
- Freeland-Graves, J.H.; Mousa, T.Y.; Sanjeevi, N. Nutritional requirements for manganese. In Manganese in Heath and Disease; Costa, G.L., Aschner, M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 34–78. [Google Scholar]
- Himeno, S.; Yanagiya, T.; Fujishiro, H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 2009, 91, 1218–1222. [Google Scholar] [CrossRef]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef]
- Ismail-Khan, R.; Bui, M.M. A review of triple-negative breast cancer. Cancer Control 2010, 17, 173–176. [Google Scholar] [CrossRef]
- Wu, H.D.; Chou, S.Y.; Chen, D.R.; Kuo, H.W. Differentiation of serum levels of trace elements in normal and malignant breast patients. Biol. Trace Elem. Res. 2006, 113, 9–18. [Google Scholar] [CrossRef]
- Choi, R.; Kim, M.J.; Sohn, I.; Kim, S.; Kim, I.; Ryu, J.M.; Choi, H.J.; Kim, J.M.; Lee, S.K.; Yu, J.; et al. Serum trace elements and their associations with breast cancer subgroups in Korean breast cancer patients. Nutrients 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Dearth, R.K.; Hiney, J.K.; Srivastava, V.K.; Hamilton, A.M.; Dees, W.L. Prepubertal exposure to elevated manganese results in estradiol regulated mammary gland ductal differentiation and hyperplasia in female rats. Exp. Biol. Med. 2014, 239, 871–882. [Google Scholar] [CrossRef]
- Luparello, C.; Romanotto, R.; Tipa, A.; Sirchia, R.; Olmo, N.; López de Silanes, I.; Turnay, J.; Lizarbe, M.A.; Stewart, A.F. Midregion parathyroid hormone-related protein inhibits growth and invasion in vitro and tumorigenesis in vivo of human breast cancer cells. J. Bone Miner. Res. 2001, 16, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Feliciano, C.; Tyner, A.L. A new method for determining the status of p53 in tumor cell lines of different origin. Oncol. Res. 2003, 13, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, M.; Loikkanen, J.; Myllynen, P.; Vähäkangas, K.H. Characterization of human breast cancer cell lines for the studies on p53 in chemical carcinogenesis. Toxicol. In Vitro 2011, 25, 1007–1017. [Google Scholar] [CrossRef]
- Lymburner, S.; McLeod, S.; Purtzki, M.; Roskelley, C.; Xu, Z. Zinc inhibits magnesium-dependent migration of human breast cancer MDA-MB-231 cells on fibronectin. J. Nutr. Biochem. 2013, 24, 1034–1040. [Google Scholar] [CrossRef]
- Ju, H.; Li, Y.; Xing, X.; Miao, X.; Feng, Y.; Ren, Y.; Qin, J.; Liu, D.; Chen, Z.; Yang, Z. Manganese-12 acetate suppresses the migration, invasion, and epithelial-mesenchymal transition by inhibiting Wnt/β-catenin and PI3K/AKT signaling pathways in breast cancer cells. Thorac. Cancer 2018, 9, 353–359. [Google Scholar] [CrossRef]
- Martin, P.; Fareh, M.; Poggi, M.C.; Boulukos, K.E.; Pognonec, P. Manganese is highly effective in protecting cells from cadmium intoxication. Biochem. Biophys. Res. Commun. 2006, 351, 294–299. [Google Scholar] [CrossRef]
- Noël, L.; Huynh-Delerme, C.; Guérin, T.; Huet, H.; Frémy, J.M.; Kolf-Clauw, M. Cadmium accumulation and interactions with zinc, copper, and manganese, analysed by ICP-MS in a long-term Caco-2 TC7 cell model. Biometals 2006, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Kikuchi, M.; Wisedpanichkij, R.; Li, B.; Takeda, K.; Na-Bangchang, K.; Moore, M.R.; Hirayama, K.; Shibahara, S. Prevention of cadmium accumulation in retinal pigment epithelium with manganese and zinc. Exp. Eye Res. 2008, 87, 587–593. [Google Scholar] [CrossRef]
- Sirchia, R.; Longo, A.; Luparello, C. Cadmium regulation of apoptotic and stress response genes in tumoral and immortalized epithelial cells of the human breast. Biochimie 2008, 90, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Effects of cadmium chloride on some mitochondria-related activity and gene expression of human MDA-MB231 breast tumor cells. J. Inorg. Biochem. 2008, 102, 1668–1676. [Google Scholar] [CrossRef]
- Casano, C.; Agnello, M.; Sirchia, R.; Luparello, C. Cadmium effects on p38/MAPK isoforms in MDA-MB231 breast cancer cells. Biometals 2010, 23, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Milne, D.B.; Sims, R.L.; Ralston, N.V. Manganese content of the cellular components of blood. Clin. Chem. 1990, 36, 450–452. [Google Scholar] [PubMed]
- Klein, L.D.; Breakey, A.A.; Scelza, B.; Valeggia, C.; Jasienska, G.; Hinde, K. Concentrations of trace elements in human milk: Comparisons among women in Argentina, Namibia, Poland, and the United States. PLoS ONE 2017, 12, e0183367. [Google Scholar] [CrossRef]
- Mulay, I.L.; Roy, R.; Knox, B.E.; Suhr, N.H.; Delaney, W.E. Trace-metal analysis of cancerous and noncancerous human tissues. J. Natl. Cancer Inst. 1971, 47, 1–13. [Google Scholar]
- Abdelkarim, M.; Vintonenko, N.; Starzec, A.; Robles, A.; Aubert, J.; Martin, M.L.; Mourah, S.; Podgorniak, M.P.; Rodrigues-Ferreira, S.; Nahmias, C.; et al. Invading basement membrane matrix is sufficient for MDA-MB-231 breast cancer cells to develop a stable in vivo metastatic phenotype. PLoS ONE 2011, 6, e23334. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St. Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.; Loo, S.Y.; Shin, S.W.; Tan, T.Z.; Eng, C.B.; Singh, R.; Putti, T.C.; Ong, C.W.; Salto-Tellez, M.; Goh, B.C.; et al. Manganese superoxide dismutase is a promising target for enhancing chemosensitivity of basal-like breast carcinoma. Antioxid. Redox Signal. 2014, 20, 2326–2346. [Google Scholar] [CrossRef] [PubMed]
- Kattan, Z.; Minig, V.; Leroy, P.; Dauça, M.; Becuwe, P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res. Treat. 2008, 108, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Starcevic, S.L.; Diotte, N.M.; Zukowski, K.L.; Cameron, M.J.; Novak, R.F. Oxidative DNA damage and repair in a cell lineage model of human proliferative breast disease (PBD). Toxicol. Sci. 2003, 75, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ghneim, H.K. The kinetics of the effect of manganese supplementation on SOD2 activity in senescent human fibroblasts. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1866–1880. [Google Scholar] [PubMed]
- Qin, S.; Liao, X.; Lu, L.; Zhang, L.; Xi, L.; Guo, Y.; Luo, X. Manganese enhances the expression of the manganese superoxide dismutase in cultured primary chick embryonic myocardial cells. J. Integr. Agric. 2017, 16, 2038–2046. [Google Scholar] [CrossRef]
- Thongphasuk, J.; Oberley, L.W.; Oberley, T.D. Induction of superoxide dismutase and cytotoxicity by manganese in human breast cancer cells. Arch. Biochem. Biophys. 1999, 365, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Ranganathan, A.C.; Mansouri, J.; Rodriguez, A.M.; Providence, K.M.; Rutter, J.L.; Pumiglia, K.; Bennett, J.A.; Melendez, J.A. Elevated sod2 activity augments matrix metalloproteinase expression: Evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin. Cancer Res. 2003, 9, 424–432. [Google Scholar] [PubMed]
- Connor, K.M.; Hempel, N.; Nelson, K.K.; Dabiri, G.; Gamarra, A.; Belarmino, J.; Van De Water, L.; Mian, B.M.; Melendez, J.A. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007, 67, 10260–10267. [Google Scholar] [CrossRef]
- Loo, S.Y.; Hirpara, J.L.; Pandey, V.; Tan, T.Z.; Yap, C.T.; Lobie, P.E.; Thiery, J.P.; Goh, B.C.; Pervaiz, S.; Clément, M.V.; et al. Manganese superoxide dismutase expression regulates the switch between an epithelial and a mesenchymal-Like phenotype in breast carcinoma. Antioxid. Redox Signal. 2016, 25, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D. Protective effect of manganese in cadmium-induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip. Toxicol. 2010, 3, 68–72. [Google Scholar] [CrossRef]
- Chaudhary, S.; Iram, S.; Raisuddin, S.; Parvez, S. Manganese pre-treatment attenuates cadmium induced hepatotoxicity in Swiss albino mice. J. Trace Elem. Med. Biol. 2015, 29, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Isaev, N.K.; Avilkina, S.; Golyshev, S.A.; Genrikhs, E.E.; Alexandrova, O.P.; Kapkaeva, M.R.; Stelmashook, E.V. N-acetyl-l-cysteine and Mn2+ attenuate Cd2+-induced disturbance of the intracellular free calcium homeostasis in cultured cerebellar granule neurons. Toxicology 2018, 393, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Longo, A.; Vetrano, M. Exposure to cadmium chloride influences astrocyte-elevated gene-1 (AEG-1) expression in MDA-MB231 human breast cancer cells. Biochimie 2012, 94, 207–213. [Google Scholar] [CrossRef]
- Pan, C.; Liu, H.D.; Gong, Z.; Yu, X.; Hou, X.B.; Xie, D.D.; Zhu, X.B.; Li, H.W.; Tang, J.Y.; Xu, Y.F.; et al. Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Sci. Rep. 2013, 3, 2333. [Google Scholar] [CrossRef]
- Caradonna, F.; Luparello, C. Cytogenetic characterization of HB2 epithelial cells from the human breast. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 48–55. [Google Scholar] [CrossRef]
- Luparello, C.; Librizzi, M.; Asaro, D.M.L.; Cruciata, I.; Caradonna, F. Mid-region parathyroid hormone-related protein is a genome-wide chromatin-binding factor that promotes growth and differentiation of HB2 epithelial cells from the human breast. Biofactors 2018. [Google Scholar] [CrossRef]
- Cristal Violet Assay. Available online: http://www2.kumc.edu/soalab/LabLinks/protocols/cvassay.htm (accessed on 8 July 2018).
- Liu, F.; Verin, A.D.; Wang, P.; Day, R.; Wersto, R.P.; Chrest, F.J.; English, D.K.; Garcia, J.G. Differential regulation of sphingosine-1-phosphate- and VEGF-induced endothelial cell chemotaxis. Involvement of Giα2-linked Rho kinase activity. Am. J. Respir. Cell Mol. Biol. 2001, 24, 711–719. [Google Scholar] [CrossRef]
- Development of Cancer Cell Invasion Assays in a 96 Well Format. Available online: http://www.emdmillipore.com/Web-PR-Site/en_CA/-/USD/ShowDocument-File?ProductSKU=MM_NF-C7748&DocumentId=201306.9636.ProNet&DocumentUID=9950893&DocumentType=PO&Language=EN&Country=NF&Origin=PDP (accessed on 8 July 2018).
- Blaurock-Busch, E.; Busch, Y.M.; Friedle, A.; Buerner, H.; Parkash, C.; Kaur, A. Comparing the metal concentration in the hair of cancer patients and healthy people living in the Malwa region of Punjab, India. Clin. Med. Insights Oncol. 2014, 8, 1–13. [Google Scholar] [CrossRef]
- White, A.J.; Weinberg, C.R.; O’Meara, E.S.; Sandler, D.P.; Sprague, B.L. Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res. 2019, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Cerrillo, V.; Matesanz, A.I.; Millán, J.M.; Navarro, P.; Alonso, C.; Souza, P. DNA interstrand cross-linking efficiency and cytotoxic activity of novel cadmium(II)-thiocarbodiazone complexes. Chembiochem 2001, 2, 119–123. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, J.; Shahzad, M.; Han, Z.; Wang, Z.; Li, J.; Sjölinder, H. Zinc supplementation protects against cadmium accumulation and cytotoxicity in Madin-Darby bovine kidney cells. PLoS ONE 2014, 9, e103427. [Google Scholar] [CrossRef]
- Rukka, N.S.; Kuzmina, L.G.; Albov, D.V.; Shamsiev, R.S.; Mudretsova, S.N.; Davydova, G.A.; Retivov, V.M.; Volkov, P.A.; Kravchenko, V.V.; Apryshko, G.N.; et al. Synthesis, X-ray crystal structure and cytotoxicity studies of zinc(II) and cadmium(II) iodide complexes with antipyrine. Polyhedron 2015, 102, 152–162. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luparello, C. Effect of Manganese Chloride and of Cotreatment with Cadmium Chloride on the In Vitro Proliferative, Motile, and Invasive Behavior of MDA-MB231 Breast Cancer Cells. Molecules 2019, 24, 1205. https://doi.org/10.3390/molecules24071205
Luparello C. Effect of Manganese Chloride and of Cotreatment with Cadmium Chloride on the In Vitro Proliferative, Motile, and Invasive Behavior of MDA-MB231 Breast Cancer Cells. Molecules. 2019; 24(7):1205. https://doi.org/10.3390/molecules24071205
Chicago/Turabian StyleLuparello, Claudio. 2019. "Effect of Manganese Chloride and of Cotreatment with Cadmium Chloride on the In Vitro Proliferative, Motile, and Invasive Behavior of MDA-MB231 Breast Cancer Cells" Molecules 24, no. 7: 1205. https://doi.org/10.3390/molecules24071205
APA StyleLuparello, C. (2019). Effect of Manganese Chloride and of Cotreatment with Cadmium Chloride on the In Vitro Proliferative, Motile, and Invasive Behavior of MDA-MB231 Breast Cancer Cells. Molecules, 24(7), 1205. https://doi.org/10.3390/molecules24071205