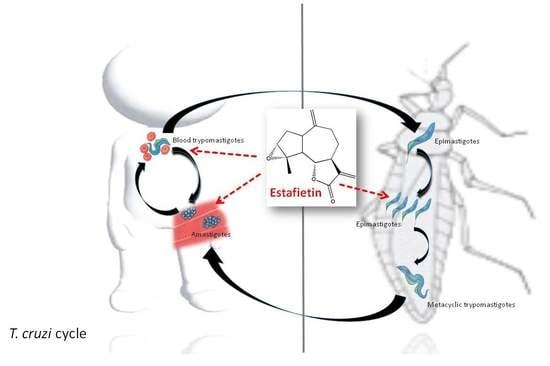
Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Biology
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of Estafietin (1)
4.3. Synthesis of Estafietin Derivatives
4.3.1. 11βH,13-Dihydroestafietin (2)
4.3.2. α-10(14)-epoxyestafietin (3a) and β-10(14)-epoxyestafietin (3b)
4.3.3. 11βH,13-Methoxyestafietin (4)
4.3.4. 11βH,13-Cianoestafietin (5)
4.4. Parasites
4.5. In Vitro Trypanocidal Activity
4.6. In Vitro Leishmanicidal Activity
4.7. Host Cell Toxicity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sülsen, V.; Martino, V. Overview. In Sesquiterpene Lactones. Advances in their Chemistry and Biological Aspects; Sülsen, V., Martino, V., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–17. [Google Scholar]
- Chaturvedi, D. Sesquiterpene lactones: Structural diversity and their biological activities. In Opportunity, Challanges and Scope of Natural Products in Medicinal Chemistry; Tiwari, V.K., Mishra, B.B., Eds.; Research Signpost: Kerala, India, 2011; pp. 313–334. [Google Scholar]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Waqstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Frank, F.M.; Cazorla, S.I.; Anesini, C.A.; Malchiodi, E.L.; Freixa, B.; Martino, V.S. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob. Agents Chemother. 2008, 52, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Frank, F.M.; Cazorla, S.I.; Barrera, P.; Freixa, B.; Vila, R.; Sosa, M.A.; Malchiodi, E.L.; Muschietti, L.V.; Martino, V.S. Psilostachyin C: A natural compound with trypanocidal activity. Int. J. Antimicrob. Agents. 2011, 37, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Sülsen, V.P.; Cazorla, S.I.; Frank, F.M.; Laurella, L.C.; Muschietti, L.V.; Catalan, C.A.; Malchiodi, E.L. Natural terpenoids from Ambrosia species are active in vitro and in vivo against human pathogenic trypanosomatids. PLoS Negl. Trop. Dis. 2013, 7, e2494. [Google Scholar] [CrossRef] [PubMed]
- Laurella, L.C.; Cerny, N.; Bivona, A.E.; Sanchez Alberti, A.; Giberti, G.; Malchiodi, E.L.; Martino, V.S.; Catalan, C.A.; Alonso, M.R.; Cazorla, S.I.; et al. Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp. PLoS Negl. Trop. Dis. 2017, 11, e0005929. [Google Scholar]
- Amorim, M.H.; Gil da Costa, R.M.; Lopes, C.; Bastos, M.M. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Sass, D.C.; Oliveira Morais, G.; Crema Miranda, R.A.; Guidi Magalhães, L.; Cunha, W.-R.; Alves dos Santos, R.; Syogo Arakawa, N.; Batista Da Costa, F.; Gomes Constantinoa, M.; Gomes Heleno, V.C. Structurally modified natural sesquiterpene lactones constitute effective and less toxic schistosomicidal compounds. Org. Biomol. Chem. 2014, 12, 7957–7964. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M. Chemical modifications of arglabin and biological activity of its new derivatives. Fitoterapia 2016, 110, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Khlebnikov, A.I.; Schepetkin, I.A.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Kirpotina, L.N.; Quinn, M.T. Inhibition of T Cell Receptor Activation by Semi-Synthetic Sesquiterpene Lactone Derivatives and Molecular Modeling of Their Interaction with Glutathione and Tyrosine Kinase ZAP-70. Molecules 2019, 24, 350. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Chagas Disease (American Tripanosomiasis). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 23 January 2019).
- World Health Organization (WHO). Leishmaniasis. 2018. Available online: https://www.who.int/leishmaniasis/en/ (accessed on 23 January 2019).
- Locatelli, F.M.; Cajal, S.P.; Barroso, P.A.; Lauthier, J.J.; Mora, M.C.; Juarez, M.; Kato, H.; Nasser, J.R.; Hashiguchi, Y.; Korenaga, M.; Marco, J.D. The isolation and molecular characterization of Leishmania spp. from patients with American tegumentary leishmaniasis in northwest Argentina. Acta Trop. 2014, 131, 16–21. [Google Scholar] [CrossRef] [PubMed]
- De Heluani, C.S.; de Lampasona, M.P.; Catalán, C.A.N.; Goedken, V.L.; Gutierrez, A.B.; Herz, W. Guaianolides, heliangolides and other constituents from Stevia alpina. Phytochemistry. 1989, 28, 1931–1935. [Google Scholar] [CrossRef]
- Fabian, L.; Sülsen, V.; Frank, F.; Cazorla, S.; Malchiodi, E.; Martino, V.; Lizarraga, E.; Catalán, C.; Moglioni, A.; Muschietti, L.; Finkielsztein, L. In Silico Study of Structural and Geometrical Requirements of Natural Sesquiterpene Lactones with Trypanocidal Activity. Mini-Rev. Med. Chem. 2013, 13, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, E.L.; Chiaramonte, M.G.; Taranto, N.J.; Zwirner, N.W.; Margni, R.A. Cross reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp: Use of immunoblotting and ELISA with a purified antigen (Ag 163B6). Clin. Exp. Immunol. 1994, 97, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Zwirner, N.W.; Caropresi, S.L.; Taranto, N.J.; Malchiodi, E.L. Trypanosoma cruzi and Leishmania spp. human mixed infection. Am. J. Trop. Med. Hyg. 1996, 54, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.G.; Frank, F.M.; Furer, G.M.; Taranto, N.J.; Margni, R.A.; Malchiodi, E.L. Polymerase chain reaction reveals T. cruzi infection suspected by serology in cutaneous and mucocutaneous leishmaniasis patients. Acta Trop. 1999, 72, 295–308. [Google Scholar] [CrossRef]
- Padilla Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Mercado, M.I.; Coll Aráos, M.V.; Grau, A.; Catalán, C.A.N. New acyclic diterpenic acids from yacon (Smallanthus sonchifolius) leaves. Nat. Prod. Commun. 2010, 5, 1721–1726. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites express-ing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of selected compounds are available from the authors. |




| Compounds | CC50 (µg/mL) | Selectivity Index | ||
|---|---|---|---|---|
| Trypomastigotes T. cruzi | Amastigotes T. cruzi | Promastigotes L. braziliensis | ||
| 1 | 240.4 | 9.5 | 8.3 | 240.4 |
| 2 | 164.6 | 1.7 | 2.0 | 126.6 |
| 3 | 8.1 | 0.4 | 4.0 | 1.0 |
| 4 | 315.6 | 3.1 | 10.3 | 6.1 |
| 5 | 133.6 | 1.7 | 1.3 | 2.7 |
| H | 1 | 2 | 3b | 4 | 5 |
|---|---|---|---|---|---|
| δ (J in Hz) | δ (J in Hz) | δ (J in Hz) | δ (J in Hz) | δ (J in Hz) | |
| 1 | 2.98 ddd | 2.90 ddd | 2.22 ddd | 2.91 ddd | 2.93 ddd |
| (10.5, 8.5, 7.5) | (10.5, 8.5, 7.3) | (10.5, 8.3, 8) | (10.5, 8.5, 7.3) | (10.5, 8, 7.3) | |
| 2a | 2.07 dd | 2.10 dd | 2.10 dd | 2.10 dd | 2.15 dd |
| (14, 7.5) | (14, 7) | (13.8, 8) | (13.9, 7.3) | (13.9, 7.3) | |
| 2b | 1.81 ddd | 1.80 ddd | 1.80 ddd | 1.80 ddd | 1.80 ddd |
| (14, 10.5, 1.2) | (14, 10.5, 1.2) | (13.8, 10.5, 1.5) | (13.9, 10.5, 1.2) | (13.9, 10.5, 0.7) | |
| 3 | 3.38 s br | 3.36 s br | 3.36 s br | 3.36 s br | 3.37 s br |
| 5 | 2.32 dd | 2.28 dd | 2.43 dd | 2.32 dd | 2.35 dd |
| (11, 8.5) | (10.7, 8.4) | (11, 8.3) | (10.7, 8.5) | (10.7, 8) | |
| 6 | 4.08 dd | 3.96 dd | 4.12 dd | 3.99 dd | 4.09 dd |
| (11, 8.8) | (10.5, 9.7) | (11, 9) | (10.7, 9.4) | (10.7, 9.5) | |
| 7 | 2.87 ddddd | 1.91 m | 2.83 m | 2.36 m | 2.27 m |
| (11.5, 8.8, 5.2, 3.6, 3.2) | |||||
| 8a | 2.22 m | 2.09 m | 2.10 m | 2.17 m | 2.34 m |
| 8b | 1.53m | 1.35 m | 1.60 m | 1.38 m | 1.51 m |
| 9a | 2.28 m | 2.29 m | 2.00 m | 2.26 m | 2.32 m |
| 9b | 2.19 m | 2.12 m | 1.60m | 2.14 m | 2.20m |
| 11 | --- | 2.22 dq | --- | 2.41ddd | 2.58 ddd |
| (12, 7) | (11.7, 4.7, 3.4) | (11.8, 7.8, 4.4) | |||
| 13a | 6.21 d | 1.22 ʘ d (7) | 6.23 d | 3.66 * dd | 2.87 * dd |
| (3.6) | (3.5) | (9.7, 4.7) | (17.2, 4.4) | ||
| 13b | 5.48 d | 5.51 d | 3.63 * dd | 2.63 * dd | |
| (3.2) | (3.1) | (9.7, 3.4) | (17.2, 7.8) | ||
| 14a | 4.95 s br | 4.88 s br | 2.64 * d | 4.89 s br | 4.93 s br |
| (4.8) | |||||
| 14b | 4.86 d | 4.83 s br | 2.61 * d | 4.84 s br | 4.88 s br |
| (1.7) | (4.8) | ||||
| 15 ʘ | 1.62 s | 1.59 s | 1.63 s | 1.58 s | 1.58 s |
| Others | --- | --- | --- | 3.36 s (OMe) | --- |
| C | 1 | 2 | 3b (*) | 4 | 5 |
|---|---|---|---|---|---|
| δ | δ | δ | δ | δ | |
| 1 | 44.9 d | 44.2 d | 42.5 d | 44.3 d | 44.2 d |
| 2 | 33.0 t | 32.7 t | 28.5 t | 32.8 t | 32.6 t |
| 3 | 63.2 d | 63.1 d | 62.8 d | 63.2 d | 63.0 d |
| 4 | 65.8 s | 66.0 s | 65.7 s | 66.1 s | 65.8 s |
| 5 | 50.8 d | 50.4 d | 50.3 d | 50.3 d | 50.2 d |
| 6 | 80.5 d | 80.8 d | 79.9 d | 80.8 d | 81.1 d |
| 7 | 44.1 d | 50.0 d | 47.3 d | 44.7 d | 47.5 d |
| 8 | 29.2 t | 30.9 t | 24.2 t | 31.2 t | 31.0 t |
| 9 | 28.6 t | 31.0 t | 24.2 t | 30.9 t | 30.4 t |
| 10 | 146.1 s | 147.3 s | 57.1 s | 147.2 s | 146.3 s |
| 11 | 139.6 s | 41.9 d | 139.2 s | 47.9 d | 43.6 d |
| 12 | 169.7 s | 178.4 s | 169.5 s | 175.8 s | 174.1 s |
| 13 | 120.2 t | 13.2 q | 120.2 t | 69.2 t | 17.1 t |
| 14 | 115.3 t | 114.1 t | 53.1 t | 114.2 t | 114.9 t |
| 15 | 18.5 q | 18.7 q | 19.0 q | 18.7 q | 18.6 q |
| Others | --- | --- | --- | 53.9 (q; OMe) | 116.7 (s; CN) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sülsen, V.P.; Lizarraga, E.F.; Elso, O.G.; Cerny, N.; Sanchez Alberti, A.; Bivona, A.E.; Malchiodi, E.L.; Cazorla, S.I.; Catalán, C.A.N. Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 2019, 24, 1209. https://doi.org/10.3390/molecules24071209
Sülsen VP, Lizarraga EF, Elso OG, Cerny N, Sanchez Alberti A, Bivona AE, Malchiodi EL, Cazorla SI, Catalán CAN. Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules. 2019; 24(7):1209. https://doi.org/10.3390/molecules24071209
Chicago/Turabian StyleSülsen, Valeria P., Emilio F. Lizarraga, Orlando G. Elso, Natacha Cerny, Andrés Sanchez Alberti, Augusto E. Bivona, Emilio L. Malchiodi, Silvia I. Cazorla, and César A. N. Catalán. 2019. "Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis" Molecules 24, no. 7: 1209. https://doi.org/10.3390/molecules24071209
APA StyleSülsen, V. P., Lizarraga, E. F., Elso, O. G., Cerny, N., Sanchez Alberti, A., Bivona, A. E., Malchiodi, E. L., Cazorla, S. I., & Catalán, C. A. N. (2019). Activity of Estafietin and Analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules, 24(7), 1209. https://doi.org/10.3390/molecules24071209