3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from Bark of Eysenhardtia polystachya Prevents Diabetic Nephropathy via Inhibiting Protein Glycation in STZ-Nicotinamide Induced Diabetic Mice
Abstract
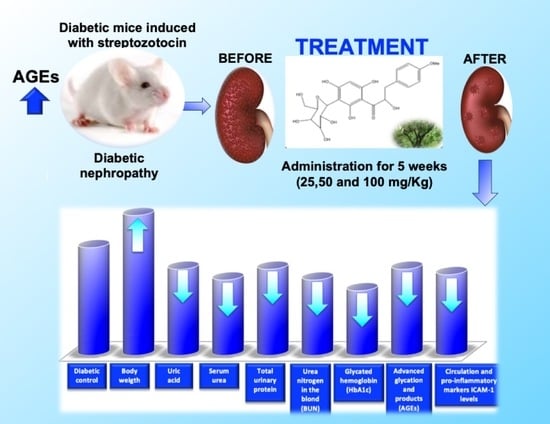
:1. Introduction
2. Results and Discussion
2.1. Identification of 3′-O-β-d-glucopyranosyl α,4,2′,4′,6′-pentahydroxy–dihydrochalcone (1)
2.2. Effect of Dihydrochalcone on Glucose, Water Intake, Body Weight, Kidney Weight, Food Consumption, Urine Volumen and Urine Protein
2.3. Effect of Dihydrochalcone on Kidney Index, Creatinine, Uric Acid, Serum Urea, and Urea Nitrogen in the Blood (BUN)
2.4. Effect of Dihydrochalcone on MCP-1
2.5. Effect of Dihydrochalcone on Glycosylated Haemoglobin Concentration (HbA1c)
2.6. Effect of Dihydrochalcone on AGEs Kidney
2.7. AGEs Levels in Plasma
2.8. Histopathology on in Renal Tissues
3. Conclusions
4. Materials and Methods
4.1. Plant
4.2. Extraction, Isolation and Characterization 3′-O-β-d-glucopyranosyl α,4,2′,4′,6′-pentahydroxy–dihydrochalcone (1)
4.3. Animals
4.4. Induction of Mild Diabetes (Type 2)
4.5. Preparation of Urine, Serum, and Kidney Homogenate
4.6. Measured of Biochemical Parameters in Diabetic Mice
4.7. Glycosylated Hemoglobin
4.8. AGEs Levels in Kidney
4.9. AGEs Levels in Serum
4.10. Measure of the Expression of Intracellular Adhesion Molecule (ICAM)-1
4.11. Histopathology
4.12. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Yan, H.D.; Li, X.Z.; Xie, J.M.; Li, M. Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells. Chin. Med. J. 2007, 120, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A. Prevention of loss of renal function over time in patients with diabetic nephropathy. Am. J. Med. 2006, 119, S40–S47. [Google Scholar] [CrossRef]
- Babaei-Jadidi, R.; Karachalias, N.; Ahmed, N.; Battah, S.; Thornalley, P. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 2003, 52, 2110–2120. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thallas, V.; Thomas, M.C. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003, 17, 1762–1764. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Bolton, W.K.; Khalifah, R.G.; Degenhardt, T.P.; Schotzinger, R.J.; McGill, J.B. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 2007, 27, 605–614. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J.; Cai, W.; Striker, G. Advanced glycation end product homeostasis: Exogenous oxidants and innate defenses. Ann. N. Y. Acad. Sci. 2008, 1126, 46–52. [Google Scholar] [CrossRef]
- Stinghen, A.E.; Goncalves, S.M.; Martines, E.G. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron. Clin. Pract. 2009, 111, 117–126. [Google Scholar] [CrossRef]
- Sanajou, D.; Haghjo, A.G.; Argani, H.; Aslani, S. Age-rage axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–166. [Google Scholar] [CrossRef]
- Perez, R.M.G.; Garcia, A.H.C.; Mota, J.M.F. Dihydrochalcones from the bark of Eysenhardtia polystachya inhibit formation of advanced glycation and products at multiple stages in vitro studies. NESSA J. Pharm. Pharm. 2017, 1, 3–23. [Google Scholar]
- Madeline, J.; Simpson, I.; Hjelmqvist, D.; López-A, C.A.; Karamehmedovic, N.; Minehan, T.G.; Yepremyan, A.; Salehani, B.; Lissi, E.; Joubert, E.; et al. Anti-Peroxyl radical quality and antibacterial properties of rooibos infusions and their pure glycosylated polyphenolic constituents. Molecules 2013, 18, 11264–11280. [Google Scholar] [CrossRef]
- Tesch, G.H.; Allen, T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 2007, 12, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic Potential of Nrf2 Activators in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Walmsley, S.J.; Broeckling, C.; Hess, A.; Prenni, J.; Curthoys, N.P. Proteomic Analisis of brush-border membrane vesicles isolated from purified proximal convoluted tubules. Am. J. Physiol.-Ren. Physiol. 2010, 298, F1323–F1331. [Google Scholar] [CrossRef]
- Dollah, M.A.; Parhizkar, S.; Izwan, M. Effect of Nigella sativa on the kidney function in rats. Avicenna J. Phytomed. 2013, 3, 152–156. [Google Scholar]
- Huang, M.; Liang, Q.; Li, P. Biomarkers for early diagnosis of type 2 diabetic nephropathy: A study based on an integrated biomarker system. Mol. BioSyst. 2013, 9, 2134–2141. [Google Scholar] [CrossRef]
- Sassy-Prigent, C.; Heudes, D.; Mandet, C. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 2000, 49, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.; Ray, R.; Arya, D.S. Chrysin, a PPAR-y agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef]
- Gobbay, K.H. Glycosylated haemogobin and diabetic control. N. Eng. J. Med. 1976, 295, 443–444. [Google Scholar] [CrossRef]
- Rahbar, S.; Figarola, J.L. Novel inhibitors of advanced glycation end products. Arch. Biochem. Biophys. 2003, 419, 63–79. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Heidari, H.; Oroojan, A.A.; Mirzavandi, F.; Nasr, K.E.; Dehghan, Z.M. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 2017, 7, 169–179. [Google Scholar] [PubMed]
- Parker, K.M.; England, J.K.; Da-Costa, J.; Hess, R.L.; Goldstein, D.E. Improved colorimetric assay for glycosylated hemoglobin. Clin. Chem. 1981, 27, 669–672. [Google Scholar] [PubMed]
- Kalousova, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [PubMed]
- Sampathkumar, R.; Balasubramanyam, M.; Rema, M.; Premanand, C.; Mohan, V. Novel advanced glycation index and its association with diabetes and microangiopathy. Metabolism 2005, 54, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Sciacqua, A.; Andreozzi, F.; Perticone, F.; Sesti, G. Elevated hemoglobin glycation index identify non-diabetic individuals at increased risk of kidney dysfunction. Oncotarget 2017, 8, 79576–79586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






| Groups | Urine Protein (mg/dL) | |
|---|---|---|
| 0 Weeks | 5 Weeks | |
| NormaL ControL | 0.45 ± 0.03 a | 0.44 ± 0.08 a |
| Diabetic ControL | 2.82 ± 0.7 | 3.61 ± 0.4 |
| D + 1 (25 mg/dL) | 2.81 ± 0.9 | 2.69 ± 0.5 |
| D + 1 (50 mg/dL) | 2.88 ± 0.6 | 2.16 ± 0.7 |
| D + 1 (100 mg/dL) | 2.89 ± 1.0 | 1.72 ± 0.9 a |
| D + Met (200 mg/dL) | 2.80 ± 1.3 | 1.70 ± 0.6 a |
| Groups | BUN (mg/dL) | Creatinine (mg/dL) | Uric Acid (mg/dL) | Urea (mg/dL) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 Weeks | 5 Weeks | 0 Weeks | 5 Weeks | 0 Weeks | 5 Weeks | 0 Weeks | 5 Weeks | |
| NormaL ControL | 15.89 ± 3.4 a | 16.34 ± 2.8 a | 0.73 ± 0.03 a | 0.073 ± 0.03 a | 5.12 ± 1.4 a | 5.11 ± 1.0 a | 35.06 ± 4.3 a | 36.49 ± 1.0 a |
| Diabetic ControL | 36.12 ± 4.1 | 42.51 ± 2.9 | 3.01 ± 0.9 | 3.90 ± 0.7 | 13.11 ± 3.7 | 16.20 ± 3.1 | 80.10 ± 5.9 | 90.38 ± 6.4 |
| D + 1 (25 mg/dL) | 38.24 ± 2.9 | 30.47 ± 3.5 a | 3.25 ± 1.0 | 2.47 ± 0.5 | 13.30 ± 2.8 | 8.13 ± 2.5 a | 82.12 ± 3.7 | 69.28 ± 5.3 a |
| D + 1 (50 mg/dL) | 37.23 ± 5.3 | 25.48 ± 4.1 a | 3.94 ± 0.8 | 2.10 ± 0.4 a | 12.94 ± 1.7 | 6.24 ± 2.9 a | 83.43 ± 4.2 | 58.19 ± 6.1 a |
| D + 1 (100 mg/dL) | 38.48 ± 4.6 | 22.11 ± 3.9 a | 3.76 ± 0.6 | 1.70 ± 0.08 a | 13.84 ± 1.5 | 5.41 ± 1.6 a | 81.71 ± 5.3 | 50.13 ± 3.7 a |
| D + Met (200 mg/dL) | 38.40 ± 5.0 | 21.43 ± 4.2 a | 3.83 ± 0.9 | 1.55 ± 0.6 a | 12.67 ± 3.6 | 3.43 ± 2.3 a | 82.84 ± 3.8 | 56.22 ± 4.6 a |
| Groups | Glycosylated Haemoglobin (%) | AGEs (RFU/mg Protein) |
|---|---|---|
| NormaL controL | 3.52 ± 0.7 a | 1.71 ± 0.9 a |
| Diabetic controL | 9.45 ± 1.1 a | 3.8 ± 0.8 |
| D + 1 (25 mg/dL) | 7.61 ± 1.8 | 3.0 ± 0.7 |
| D + 1 (50 mg/dL) | 6.27 ± 0.9 a | 2.6 ± 0.9 |
| D + 1 (100 mg/dL) | 4.3 ± 0.8 a | 2.0 ± 0.6 |
| D + Met (200 mg/dL) | 4.9 ± 0.6 a | 1.9 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Gutierrez, R.M.; García Campoy, A.H.; Paredes Carrera, S.P.; Muñiz Ramirez, A.; Mota Flores, J.M.; Flores Valle, S.O. 3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from Bark of Eysenhardtia polystachya Prevents Diabetic Nephropathy via Inhibiting Protein Glycation in STZ-Nicotinamide Induced Diabetic Mice. Molecules 2019, 24, 1214. https://doi.org/10.3390/molecules24071214
Pérez Gutierrez RM, García Campoy AH, Paredes Carrera SP, Muñiz Ramirez A, Mota Flores JM, Flores Valle SO. 3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from Bark of Eysenhardtia polystachya Prevents Diabetic Nephropathy via Inhibiting Protein Glycation in STZ-Nicotinamide Induced Diabetic Mice. Molecules. 2019; 24(7):1214. https://doi.org/10.3390/molecules24071214
Chicago/Turabian StylePérez Gutierrez, Rosa Martha, Abraham Heriberto García Campoy, Silvia Patricia Paredes Carrera, Alethia Muñiz Ramirez, José Maria Mota Flores, and Sergio Odin Flores Valle. 2019. "3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from Bark of Eysenhardtia polystachya Prevents Diabetic Nephropathy via Inhibiting Protein Glycation in STZ-Nicotinamide Induced Diabetic Mice" Molecules 24, no. 7: 1214. https://doi.org/10.3390/molecules24071214
APA StylePérez Gutierrez, R. M., García Campoy, A. H., Paredes Carrera, S. P., Muñiz Ramirez, A., Mota Flores, J. M., & Flores Valle, S. O. (2019). 3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from Bark of Eysenhardtia polystachya Prevents Diabetic Nephropathy via Inhibiting Protein Glycation in STZ-Nicotinamide Induced Diabetic Mice. Molecules, 24(7), 1214. https://doi.org/10.3390/molecules24071214