Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Analysis
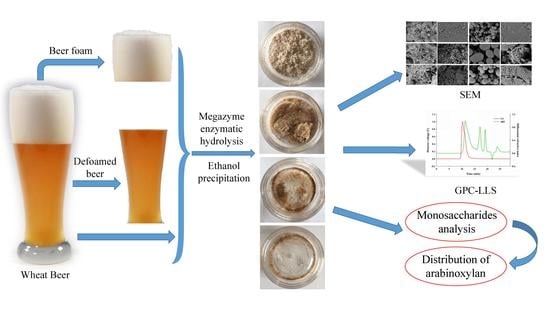
2.2. SEM Assay
2.3. Monosaccharides Composition Analysis
2.4. Analysis of Molecular Weight
3. Materials and Methods
3.1. Materials
3.2. Preparation of Samples
3.2.1. Separation of Beer Foam
3.2.2. Purification of Arabinoxylan
Step One
Step Two
Step Three
Step Four
3.3. Fractionation of Arabinoxylan by Gradient Ethanol Precipitation
3.4. Analytical Methods
3.4.1. Physico-Chemical Analysis
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. Analysis of Monosaccharide Composition and Polysaccharides
Hydrolysis
Reduction
Derivatization
Extraction
Chromatographic Condition
3.4.4. Analysis of Residual β-Glucan and Dextrin
3.4.5. Molecular Characterization
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amienyo, D.; Azapagic, A. Life cycle environmental impacts and costs of ber production and consumption in the UK. Int. J. Life Cycle Ass. 2016, 21, 492–509. [Google Scholar] [CrossRef]
- He, G.; Du, J.; Zhang, K.; Wei, G.; Wang, W. Antioxidant capability and potableness of fresh cloudy wheat beer stored at different temperatures. J. Inst. Brew. 2013, 118, 386–392. [Google Scholar] [CrossRef]
- Mares, D.J.; Stone, B.A. Studies on wheat endosperm. 1. Chemical compositions and ultrastructure of cell-walls. Aust. J. Biol. Sci. 1973, 26, 793–812. [Google Scholar]
- Delcour, J.A.; Van Win, H.; Grobet, P.J. Distribution and structural variation of arabinoxylans in common wheat mill streams. J. Agric. Food Chem. 1999, 47, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Chornick, T.; Biliaderis, C.G.; Izydorczyk, M.S. Sequential solvent extraction and structural characterization of polysaccharides from the endosperm cell walls of barley grown in different environments. Carbohyd. Polym. 2008, 73, 621–639. [Google Scholar] [CrossRef]
- Pomeranz, Y. Wheat: Chemistry and Technology; American Association of Cereal Chemists: St. Paul, MN, USA, 1998. [Google Scholar]
- Fangel, J.U.; Eiken, J.; Sierksma, A.; Schols, H.A.; Willats, W.; Harholt, J. Tracking polydaccharides through the brewing process. Carbohyd. Polym. 2018, 196, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Cyran, M.R.; Ceglinska, A. Genetic variation in the extract viscosity of rye (Secale cereale L.) bread made from endosperm and wholemeal flour: Impact of high-molecular weight AXs, starch and protein. J. Sci. Food Agric. 2011, 91, 469–479. [Google Scholar] [CrossRef]
- Li, J.; Du, J.H.; Wu, X.Y.; Zhang, Z.A.; Zhang, K.L. Changes in crude AX during cloudy wheat beer brewing on a production scale. J. Inst. Brew. 2017, 123, 192–198. [Google Scholar] [CrossRef]
- Krahl, M.; Müller, S.; Zarnkow, M.; Back, W.; Becker, T. AX and fructan in the malting and brewing process. Qual. Assur. Saf. Cr. 2009, 1, 246–255. [Google Scholar] [CrossRef]
- Guo, M.M.; Du, J.H.; Zhang, K.L.; Jin, Y.H. Content and molecular weight of water-extractable arabinoxylans in wheat malt and wheat malt-based wort with different kolbach indices. J. Sci. Food Agric. 2014, 94, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y. Effects of AX solubilization on wort viscosity and filtration when mashing with grist containing wheat and wheat malt. Food Chem. 2006, 98, 164–170. [Google Scholar] [CrossRef]
- Saeed, F.; Pasha, I.; Anjum, F.M.; Sultan, M.T. Arabinoxylans and arabinogalactans: A comprehensive treatise. Crit. Rev. Food Sci. 2011, 51, 467–476. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley β-glucans and AXs: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Faltermaier, A.; Waters, D.; Becker, T.; Arendt, E.; Gastl, M. Common wheat (Triticum aestivum L.) and its use as a brewing cereal—A review. J. Inst. Brew. 2014, 120, 1–15. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.Z.; Qian, T.X.; Sun, G.B.; Guo, Y.; Chang, F.J.; Zhou, S.M.; Sun, X.B. Antitumor and immunomodulatory activity of arabinoxylans: A major constituent of wheat bran. Int. J. Biol. Macromol. 2011, 48, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Košťálová, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohyd. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-related effects and improving extractability of cereal arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831. [Google Scholar] [CrossRef]
- Garcia, A.L.; Otto, B.; Reich, S.-C.; Weickert, M.O.; Steiniger, J.; Machowetz, A.; Rudovich, N.N.; Möhlig, M.; Katz, N.; Speth, M.; et al. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Mendis, M.; Simsek, S. Arabinoxylans and human health. Food Hydrocolloid. 2014, 42, 239–243. [Google Scholar] [CrossRef]
- Zhou, H.J.; Ma, H.L.; Guo, D.Z.; Wang, Z.B. Physicochemical properties and antioxidant activity of intracellular polysaccharides from Phellinus igniarius precipitated by different ethanol concentrations. Food Sci. 2015, 36, 34–38. [Google Scholar]
- Kang, J.; Cui, S.W.; Chen, J.; Phillips, G.O.; Wu, Y.; Wang, Q. New studies on gum ghatti (Anogeissus latifolia) part I. Fractionation, chemical and physical characterization of the gum. Food Hydrocolloid. 2011, 25, 1984–1990. [Google Scholar] [CrossRef]
- Liepman, A.H.; Nairn, C.J.; Willats, W.G.T.; Sørensen, I.; Roberts, A.W.; Keegstra, K. Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants. Plant Physiol. 2007, 143, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Verwimp, T.; Craeyveld, V.V.; Courtin, C.M.; Delcour, J.A. Variability in the structure of rye flour alkali-extractable arabinoxylans. J. Agric. Food Chem. 2007, 55, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Gruppen, H.; Hamer, R.J.; Voragen, A.G.J. Water-unextractable cell wall material from wheat flour. 2. Fractionation of alkali-extracted polymers and comparison with water-extractable arabinoxylans. J. Cereal Sci. 1992, 16, 53–67. [Google Scholar] [CrossRef]
- Tester, R.F.; Al-Ghazzewi, F.H. Mannans and health, with a special focus on glucomannans. Food Res. Int. 2013, 50, 384–391. [Google Scholar] [CrossRef]
- Trogh, I.; Croes, E.; Courtin, C.M.; Delcour, J.A. Enzymic degradability of hull-less barley flour alkali-solubilized arabinoxylan fractions by endoxylanases. J. Agric. Food Chem. 2005, 53, 7243–7250. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Smith, C.; Li, W.L. Extraction and modification technology of AXs from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal AXs: Advances in structure and physicochemical properties. Carbohyd. Polym. 1995, 28, 33–38. [Google Scholar] [CrossRef]
- Wu, X.Y.; Du, J.H.; Zhang, K.L.; Ju, Y.D.; Jin, Y.H. Changes in protein molecular weight during cloudy wheat beer brewing. J. Inst. Brew. 2014, 121, 137–144. [Google Scholar] [CrossRef]
- α-Amylase (Bacillus licheniformis). Available online: https://secure.megazyme.com/Alpha-Amylase-Bacillus-licheniformis (accessed on 25 March 2019).
- Protease (Subtilisin A from Bacillus licheniformis). Available online: https://secure.megazyme.com/Protease-Subtilisin-A-Bacillus-licheniformis (accessed on 25 March 2019).
- Lichenase (endo-1,3:1,4-β-D-Glucanase) (Bacillus subtilis). Available online: https://secure.megazyme.com/Lichenase-endo-1-3-4-Beta-D-Glucanase-Bacillus-sp (accessed on 25 March 2019).
- Amyloglucosidase (Aspergillus niger). Available online: https://secure.megazyme.com/Amyloglucosidase-Aspergillus-Niger (accessed on 25 March 2019).
- Kordali, S.; Cakir, A.; Akcinc, T.A.; Mete, E.; Adnan Akcine, A.; Aydinb, T.; Kilic, H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crop Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Anonymous. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Iravani, S.; Fitchett, C.S.; Georget, D.M.R. Physical characterization of AX powder and its hydrogel containing a methyl xanthine. Carbohyd. Polym. 2011, 85, 201–207. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- Den Bulck, K.V.; Swennen, K.; Loosveld, A.-M.A.; Courtin, C.M.; Beijs, K.; Proost, P.; Damme, J.V.; Campenhout, S.V.; Mort, A.; Delcour, J.A. Isolation of cereal arabinogalactan-peptides and structural comparison of their carbohydrate and peptide moieties. J. Cereal Sci. 2005, 41, 59–67. [Google Scholar] [CrossRef]
- Zhang, L.N.; Zhang, X.F.; Zhou, Q.; Zhang, P.Y.; Zhang, M.; Li, X.L. Triple helix of β-D-Glucan from Lentinus Edodes in 0.5M NaCl aqueous solution characterized by light scattering. Polymer 2001, 33, 317–321. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Fractions | B | BF | DB |
|---|---|---|---|
| Yield 1 (%) | 31.88 ± 0.37 a | 32.93 ± 0.72 a | 31.83 ± 1.10 a |
| Protein (%) | 14.2 ± 0.2 a | 14.9 ± 1.1 a | 14.9 ± 0.6 a |
| Moisture (%) | 3.6 ± 0.0 c | 4.4 ± 0.0 a | 4.0 ± 0.0 b |
| Ash (%) | 1.2 ± 0.1 a | 1.0 ± 0.2 a | 1.2 ± 0.0 a |
| Monosaccharides (mg/g) | |||
| Arabinose | 15.97 ± 0.25 b | 16.99 ± 0.16 a | 15.17 ± 0.51 b |
| Xylose | 23.11 ± 0.22 a | 22.66 ± 0.35 a | 20.50 ± 0.91 b |
| Mannose | 6.02 ± 0.49 a | 6.23 ± 0.31 a | 6.81 ± 1.27 a |
| Galactose | 5.19 ± 0.67 a | 5.16 ± 0.33 a | 5.21 ± 0.80 a |
| Glucose | 548.61 ± 3.10 b | 587.84 ± 1.40 a | 547.34 ± 3.35 b |
| Total sugar | 598.90 ± 2.83 b | 638.87 ± 1.17 a | 595.03 ± 4.13 b |
| Fractions | Yields2 (%) | Protein (%) | Monosaccharides2 (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| Arabinose | Xylose | Mannose | Galactose | Glucose | |||
| B50 | 12.56 ± 0.62 a | 9.37 ± 0.66 a | 117.39 ± 0.81 c | 227.15 ± 1.10 c | 97.33 ± 2.17 a | 6.04 ± 0.47 b | - |
| BF50 | 13.22 ± 0.99 a | 7.58 ± 1.22 a | 151.94 ± 0.52 a | 271.35 ± 1.48 a | 87.37 ± 1.33 b | 6.21 ± 0.71 b | - |
| DB50 | 14.01 ± 0.36 a | 6.11 ± 070 a | 141.55 ± 2.10 b | 257.56 ± 3.18 b | 89.28 ± 1.74 b | 8.43 ± 1.53 a | - |
| B67 | 20.15 ± 0.97 a | 10.31 ± 1.30 a | 115.91 ± 1.50 a | 111.31 ± 1.97 b | 9.54 ± 0.62 a | 55.86 ± 1.01 a | 15.37 ± 1.56 b |
| BF67 | 20.08 ± 0.59 a | 8.38 ± 0.17 a | 109.16 ± 0.43 c | 112.98 ± 0.66 ab | 10.65 ± 0.38 a | 42.61 ± 1.48 c | 23.72 ± 1.65 a |
| DB67 | 20.85 ± 0.73 a | 7.94 ± 0.05 a | 113.27 ± 0.33 b | 115.56 ± 0.57 a | 9.22 ± 1.80 a | 49.44 ± 1.55 b | 17.48 ± 3.27 b |
| B75 | 19.65 ± 0.16 a | 5.23 ± 0.28 b | 33.96 ± 0.42 c | 43.47 ± 1.99 c | 11.83 ± 1.13 a | 7.09 ± 0.07 c | 197.35 ± 3.07 b |
| BF75 | 19.85 ± 0.83 a | 5.48 ± 0.52 b | 45.40 ± 1.03 a | 57.27 ± 0.34 a | 15.08 ± 1.82 a | 8.90 ± 0.77 b | 193.10 ± 2.66 b |
| DB75 | 18.32 ± 0.62 a | 6.98 ± 0.11 a | 41.43 ± 0.66 b | 51.60 ± 0.73 b | 13.52 ± 1.03 a | 11.61 ± 0.83 a | 206.96 ± 0.79 a |
| B80 | 7.23 ± 0.16 a | 3.86 ± 0.17 a | 23.54 ± 0.82 b | 31.95 ± 1.51 a | 20.47 ± 1.64 a | 4.43 ± 1.57 a | 167.89 ± 1.22 b |
| BF80 | 7.29 ± 0.65 a | 7.91 ± 1.16 a | 22.60 ± 0.48 b | 31.00 ± 0.21 a | 13.24 ± 0.33 b | 4.41 ± 0.43 a | 174.03 ± 1.37 a |
| DB80 | 7.88 ± 0.17 a | 4.83 ± 1.30 a | 26.16 ± 1.08 a | 31.83 ± 2.59 a | 16.10 ± 3.57 ab | 6.04 ± 1.22 a | 148.51 ± 1.88 c |
| Fractions | AXP | AG (mg/g) | MP (mg/g) | GP (mg/g) | Total GP (mg/g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| AX (mg/g) | A/X | avDP | β-Glucan | Dextrin | Other GP | ||||
| B | 34.39 ± 0.31 b | 0.53 ± 0.03 a | 24.90 ± 0.54 a | 7.87 ± 1.01 a | 5.42 ± 0.44 a | 13.13 ± 0.25 b | 0.53 ± 0.03 a | 479.11 ± 2.79 b | 493.75 ± 2.79 b |
| BF | 34.89 ± 0.44 a | 0.59 ± 0.01 a | 20.48 ± 0.35 b | 7.82 ± 0.50 a | 5.61 ± 0.28 a | 12.99 ± 0.14 b | 0.59 ± 0.01 a | 514.57 ± 1.26 a | 529.05 ± 1.26 a |
| DB | 31.39 ± 1.10 b | 0.56 ± 0.04 a | 17.01 ± 0.86 c | 7.90 ± 1.22 a | 6.13 ± 1.14 a | 14.10 ± 0.28 a | 0.56 ± 0.04 a | 476.84 ± 3.02 b | 492.60 ± 3.02 b |
| B50 | 303.19 ± 1.63 c | 0.50 ± 0.00 c | 961.58 ± 5.67 b | 9.16 ± 0.71 a | 87.60 ± 1.95 a | 0.09 ± 0.01 a | 0.50 ± 0.00 c | - | - |
| BF50 | 372.49 ± 1.54 a | 0.54 ± 0.00 a | 1065.22 ± 3.83 a | 9.41 ± 1.07 a | 78.64 ± 1.20 b | 0.04 ± 0.00 b | 0.54 ± 0.00 a | - | - |
| DB50 | 351.22 ± 4.64 b | 0.53 ± 0.00 b | 625.11 ± 1.62 c | 12.78 ± 2.32 a | 80.35 ± 1.56 b | 0.05 ± 0.01 b | 0.53 ± 0.00 b | - | - |
| B67 | 199.95 ± 3.03 ab | 0.69 ± 0.01 a | 49.79 ± 0.33 c | 84.69 ± 1.52 a | 8.59 ± 0.56 a | 0.21 ± 0.02 b | 0.69 ± 0.01 a | 12.46 ± 1.40 b | 13.83 ± 1.40 b |
| BF67 | 195.49 ± 0.96 b | 0.70 ± 0.01 a | 60.29 ± 1.01 a | 64.60 ± 2.24 c | 9.59 ± 0.35 a | 2.08 ± 0.06 a | 0.70 ± 0.01 a | 18.56 ± 1.48 a | 21.34 ± 1.48 a |
| DB67 | 201.37 ± 0.79 a | 0.68 ± 0.01 a | 54.46 ± 0.74 b | 74.95 ± 2.35 b | 8.30 ± 1.62 a | 0.23 ± 0.01 b | 0.68 ± 0.01 a | 14.86 ± 2.95 ab | 15.73 ± 2.95 b |
| B75 | 68.14 ± 2.11 c | 0.67 ± 0.02 ab | 58.60 ± 1.26 c | 10.75 ± 0.11 c | 10.65 ± 1.02 a | 0.45 ± 0.04 b | 0.67 ± 0.02 ab | 175.43 ± 2.76 b | 177.61 ± 2.76 b |
| BF75 | 90.35 ± 1.21 a | 0.68 ± 0.00 a | 89.96 ± 1.89 a | 13.49 ± 1.17 b | 13.57 ± 1.64 a | 0.60 ± 0.05 b | 0.68 ± 0.00 a | 171.42 ± 2.39 b | 173.79 ± 2.39 b |
| DB75 | 81.87 ± 1.19 b | 0.65 ± 0.02 b | 68.37 ± 1.87 b | 17.60 ± 1.26 a | 12.17 ± 0.93 a | 3.69 ± 0.21 a | 0.65 ± 0.02 b | 180.76 ± 0.71 a | 186.26 ± 0.71 a |
| B80 | 48.83 ± 1.69 a | 0.64 ± 0.02 a | 38.23 ± 1.25 b | 6.71 ± 2.38 a | 18.42 ± 1.47 a | 9.04 ± 0.47 b | 0.64 ± 0.02 a | 139.50 ± 1.10 a | 151.11 ± 1.10 b |
| BF80 | 47.17 ± 0.26 a | 0.63 ± 0.02 a | 49.13 ± 0.60 a | 6.68 ± 0.64 a | 11.91 ± 0.30 c | 11.27 ± 0.53 a | 0.63 ± 0.02 a | 142.81 ± 1.24 a | 156.63 ± 1.24 a |
| DB80 | 51.03 ± 3.19 a | 0.69 ± 0.05 a | 50.21 ± 1.81 a | 9.16 ± 1.86 a | 14.49 ± 0.21 b | 7.45 ± 0.38 c | 0.69 ± 0.05 a | 123.72 ± 1.69 b | 133.66 ± 1.69 c |
| B50-80 | BF50-80 | DB50-80 | |
|---|---|---|---|
| Total AX (mg/g) | 620.11 ± 1.58 c | 705.49 ± 2.27 a | 685.48 ± 7.32 b |
| AXBF: AXDB | 1.03 ± 0.01 | ||
| Total AG (mg/g) | 111.30 ± 3.96 a | 94.18 ± 3.46 b | 114.49 ± 5.70 a |
| AGBF: AGDB | 0.82 ± 0.05 | ||
| Total MP (mg/g) | 125.26 ± 2.29 a | 113.71 ± 2.40 b | 115.31 ± 4.24 b |
| MPBF: MPDB | 0.99 ± 0.02 | ||
| Total GP (mg/g) | 340.31 ± 6.19 a | 350.40 ± 3.79 a | 334.31 ± 2.18 a |
| GPBF: GPDB | 1.05 ± 0.01 | ||
| Fractions (Da) | Peak 1 | Peak 2 | Peak1:Peak2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Mp | Mw | Mw/Mn | Mn | Mp | Mw | Mw/Mn | ||
| B50 | 124,200 | 301,000 | 482,400 | 3.9 | 32,900 | 30,700 | 34,800 | 1.0 | 94.56:5.44 |
| BF50 | 74,350 | 245,200 | 472,200 | 3.1 | 49,970 | 47,440 | 51,470 | 1.0 | 93.56:6.44 |
| DB50 | 88,120 | 300,800 | 496,000 | 5.6 | 33,880 | 37,320 | 44,350 | 1.0 | 92.64:7.36 |
| B67 | 90,210 | 41,800 | 243,000 | 2.7 | 19,940 | 22,970 | 21,770 | 1.1 | 23.40:76.60 |
| BF67 | 98,040 | 45,290 | 233,000 | 2.4 | 18,270 | 21,940 | 20,790 | 1.1 | 21.54:78.46 |
| DB67 | 88,800 | 41,470 | 203,200 | 2.3 | 20,460 | 23,870 | 22,260 | 1.1 | 25.55:74.46 |
| B75 | 364,500 | 237,800 | 558,800 | 1.5 | 3176 | 2452 | 4514 | 1.4 | 0.26:99.76 |
| BF75 | - | - | - | - | 3293 | 2329 | 3275 | 1.6 | 1.40:98.60 |
| DB75 | 46,580 | 26,480 | 91,920 | 2.0 | 2794 | 2288 | 5163 | 1.2 | 1.93:98.07 |
| B80 | 816,200 | 673,000 | 198,300 | 2.4 | 1435 | 1238 | 1727 | 1.2 | 0.19:99.81 |
| BF80 | - | - | - | - | 1704 | 1821 | 2798 | 1.2 | 0:100.00 |
| DB80 | 573,500 | 524,700 | 225,200 | 3.9 | 1395 | 1166 | 2110 | 1.5 | 0.61:99.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Du, J. Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer. Molecules 2019, 24, 1230. https://doi.org/10.3390/molecules24071230
Li J, Du J. Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer. Molecules. 2019; 24(7):1230. https://doi.org/10.3390/molecules24071230
Chicago/Turabian StyleLi, Jie, and Jinhua Du. 2019. "Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer" Molecules 24, no. 7: 1230. https://doi.org/10.3390/molecules24071230
APA StyleLi, J., & Du, J. (2019). Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer. Molecules, 24(7), 1230. https://doi.org/10.3390/molecules24071230