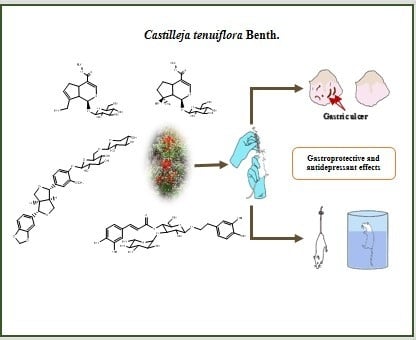
In Vivo Gastroprotective and Antidepressant Effects of Iridoids, Verbascoside and Tenuifloroside from Castilleja tenuiflora Benth
Abstract
:1. Introduction
2. Results
2.1. Chemical Identification of Isolated Compounds
2.2. Gastroprotective and Antidepressant Activity
2.2.1. Effects of Castilleja Pretreatment on CRS-Induced Gastric Damage
2.2.2. Antidepressant Effect of Integrate Extract and Fractions
2.2.3. Antidepressant Effect of Isolated Compounds
3. Materials and Methods
3.1. General Information
3.2. Extraction and Fractionation of C. Tenuiflora
3.3. Drug and C. Tenuiflora Treatments
3.4. Animals
3.5. HPLC Analysis
3.6. Pharmacological Experiments
3.6.1. Cold Restraint Stress-Induced Gastric Ulcer Test (CRS)
3.6.2. Tail Suspension Test (TST)
3.6.3. Forced Swim Test (FST)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.P.; Sung, I.K.; Kim, J.H.; Lee, S.Y.; Park, H.S.; Shim, C.S. The effect of emotional stress and depression on the prevalence of digestive diseases. J. Neurogastroenterol. Motil. 2015, 21, 273–282. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Gao, Y.; Wu, Y.; Li, Z.; Liu, H.; Zhang, C. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PLoS ONE 2012, 7, e51148. [Google Scholar] [CrossRef]
- Saxena, B.; Singh, S. Comparison of three acute stress models for simulating the pathophysiology of stress-related mucosal disease. Drug Discov. Ther. 2017, 11, 98–103. [Google Scholar] [CrossRef]
- Thornton, L.M.; Andersen, B.L.; Blakely, W.P. The pain, depression, and fatigue symptom cluster in advanced breast cancer: Covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010, 29, 333–337. [Google Scholar] [CrossRef]
- Ji, C.X.; Fan, D.S.; Li, W.; Guo, L.; Liang, Z.L.; Xu, R.M.; Zhang, J.J. Evaluation of the anti-ulcerogenic activity of the antidepressants duloxetine, amitriptyline, fluoxetine and mirtazapine in different models of experimental gastric ulcer in rats. Eur. J. Pharmacol. 2012, 691, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, F.S.; Ayoade, J.T.; Busari, A.M. Gastroprotective mechanisms of imipramine on indomethacin-induced gastric ulcer in male wistar rats. Afr. J. Biomed. Res. 2016, 19, 155–162. [Google Scholar]
- Jaganathan, R.M.; Mahendra, L.; Mahendra, J.; Kumanan, V.; Kathaperumal, K. Ethnomedical herbs and various approaches in development of new drugs. World J. Pharm. Res. 2015, 5, 869–876. [Google Scholar]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- López-Rubalcava, C.; Estrada-Camarena, E. Mexican medicinal plants with anxiolytic or antidepressant activity: Focus on preclinical research. J. Ethnopharmacol. 2016, 20, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Tank, D.C.; Olmstead, R.G. From annuals to perennials: Phylogeny of subtribe Castillejinae Orobanchaceae). Am. J. Bot. 2008, 95, 608–625. [Google Scholar] [CrossRef]
- Holmgren, N.H. Four new species of mexican Castilleja (subgenus Castilleja, Scrophulariaceae) and their relatives. Brittonia 1976, 28, 195–208. [Google Scholar] [CrossRef]
- Argueta, V.A.; Cano, A.L.M.; Rodarte, M.E. Atlas de Plantas de la Medicina Tradicional Mexicana, 1st ed.; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; Volume 2, pp. 662–663. [Google Scholar]
- Biblioteca Digital de la Medicina Tradicional Mexicana, Castilleja tenuiflora Benth. Available online: http://www.medicinatradicionalmexicana.unam.mx/monografia.php?l=3&t=Gara%C3%B1ona_o_cola_de_borrego&id=7964, 2009 (accessed on 10 January 2019).
- Sanchez, P.M.; Villarreal, M.L.; Herrera-Ruiz, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Trejo-Tapia, G. In vivo anti-inflammatory and anti-ulcerogenic activities of extracts from wild growing and in vitro plants of Castilleja tenuiflora Benth. (Orobanchaceae). J. Ethnopharmacol. 2013, 150, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; López-Rodríguez, R.; Trejo-Tapia, G.; Domínguez-Mendoza, B.E.; González-Cortazar, M.; Tortoriello, J.; Zamilpa, A. A new furofuran lignan diglycoside and other secondary metabolites from the antidepressant extract of Castilleja tenuiflora Benth. Molecules 2015, 20, 13127–13143. [Google Scholar] [CrossRef]
- Gómez-Aguirre, Y.A.; Zamilpa, A.; González-Cortazar, M.; Trejo-Tapia, G. Adventitious root cultures of Castilleja tenuiflora Benth. as a source of phenylethanoid glycosides. Ind. Crop. Prod. 2012, 36, 188–195. [Google Scholar] [CrossRef]
- López-Laredo, A.R.; Gómez-Aguirre, Y.A.; Medina-Pérez, V.; Salcedo-Morales, G.; Sepúlveda-Jiménez, G.; Trejo-Tapia, G. Variation in antioxidant properties and phenolics concentration in different organs of wild growing and greenhouse cultivated Castilleja tenuiflora Benth. Acta Physiol. Plant 2012, 34, 2435–2442. [Google Scholar] [CrossRef]
- Carrillo-Ocampo, D.; Bazalúda-Gómez, S.; Bonilla-Barbosa, J.R.; Aburto-Amar, R.; Rodríguez-López, V. Anti-inflammatory activity of iridoids and verbascoside isolated from Castilleja tenuiflora. Molecules 2013, 18, 12109–12118. [Google Scholar] [CrossRef] [PubMed]
- Demirbilek, S.; Gürses, I.; Sezgin, N.; Karaman, A.; Gürbüz, N. Protective effect of polyunsaturated phosphatidylcholind pretreatment on stress ulcer formation in rats. J. Pediatr. Surg. 2004, 39, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Adinortey, M.C.; Ansah, C.; Galyuon, I.; Nyarko, A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013, 2013, ID 796405. [Google Scholar] [CrossRef]
- Jones, J.B.; Bailey, R.T. Misoprostol: A prostaglandin E, analog with antisecretory and cytoprotective properties. DICP 1989, 23, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Yu, S.; Yan, H.; Guo, S.; Xiao, W.; Wang, Z.; Zhang, L.; Ding, A.; Wu, O.; Li, S.F.Y. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules 2017, 22, 1689. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Yamaguchi, A.; Morita, I.; Takeda, H.; Nishibe, S. Protective effects of Acanthopanax senticosus harms from Hokkaido and its components on gastric ulcer in restrained cold water stressed rat. Biol. Pharm. Bull. 1996, 19, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shukla, N.; Singh, P.; Sharma, R.; Rajendran, S.M.; Maurya, R.; Palit, G. Verbascoside isolated from Tectona grandis mediates gastric protection in rats via inhibiting proton pump activity. Fitoterapia 2010, 81, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar]
- Jaggi, A.S.; Bhatia, N.; Kumar, N.; Singh, N.; Anand, P.; Dhawan, R. A review on animal models for screening potential anti-stress agents. Neurol. Sci. 2011, 32, 993–1005. [Google Scholar] [CrossRef]
- Bauer, R.F. Misoprostol preclinical pharmacology. Digest. Dis. Sci. 1985, 30, 118S–125S. [Google Scholar] [CrossRef] [PubMed]
- Chiu, E.K.; Richardson, J.S. Behavioral and neurochemical aspects of prostaglandins in brain function. Gen. Pharmac. 1985, 16, 163–175. [Google Scholar] [CrossRef]
- Abd El-Ghffar, E.A.; Al-Sayed, E.; Shehata, S.M.; Eldahshan, O.A.; Efferth, T. The protective role of Ocimum basilicum L. (Basil) against aspirin-induced gastric ulcer in mice: Impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 2018, 9, 4457–4468. [Google Scholar] [CrossRef]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Jankord, R.; Herman, P. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. NY Acad. Sci. 2008, 1148, 64–73. [Google Scholar] [CrossRef]
- Liang, J.Q.; Wang, L.; He, J.C.; Hua, X.D. Verbascoside promotes the regeneration of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra. Neural. Regen. Res. 2016, 11, 101–106. [Google Scholar] [PubMed]
- Julião, L.S.; Leitão, S.G.; Lotti, C.; Picinelli, A.L.; Rastrelli, L.; Fernandes, P.D.; Noël, F.; Thibaut, J.P.B.; Leitão, G. Flavones and phenylpropanoids from a sedative extract of Lantana trifolia L. Phytochemistry 2010, 71, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Lin, Y.C.; Huang, T.H.; Huang, S.W.; Peng, W.H. Anti-depressive activity of Gardeniae fructus and geniposide in mouse models of depression. Afr. J. Pharm. Pharmacol. 2011, 5, 1580–1588. [Google Scholar]
- Zhao, Y.; Wang, Q.; Jia, M.; Fu, S.; Pan, J.; Chu, C.; Liu, X.; Liu, X.; Liu, Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019, 64, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Alonsoa, S.J.; Trujillo, J.; Jorge, E.; Pérez, C. Central nervous activity of elenoside. Phytomedicine 2004, 11, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, G.; Banerjee, S.; Jaiswal, D.K. New furofurano lignans from Justicia simplex. Phytochemistry 1980, 19, 332–334. [Google Scholar] [CrossRef]
- Xu, W.H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2018, 16, 1–17. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, R.K. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol. Cell Biochem. 1992, 125, 115–125. [Google Scholar] [CrossRef]
- Shuai, W.; Yong-rui, B.; Yun-Peng, D.; Xian-Sheng, M.; Ting-Guo, K. Evaluation of gastric ulcer model based on gray-scale image analysis. Afr. J. Microbiol. Res. 2011, 5, 1285–1290. [Google Scholar]
- Andrade, S.F.; Antoniolli, D.; Comunello, E.; Cardoso, L.G.V.; Carvalho, J.C.T.; Bastos, J.K. Antiulcerogenic activity of crude extract, fractions and populnoic acid isolated from Austroplenckia populnea (Celastraceae). Zeistschrift fur Naturforschung C 2006, 61, 329–333. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- González-Cortazar, M.; Maldonado-Abarca, A.M.; Jiménez-Ferrer, J.E.; Marquina, S.; Ventura-Zapata, E.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Isosakuranetin-5-O-rutinoside: A new flavanone with antidepressant activity isolated from Salvia elegans Vahl. Molecules 2013, 18, 13260–13270. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds tenuifloroside, verbascoside and mixture of geniposide/mussaenoside. are available from the authors. |







| Doses (mg/kg) | Treatments | GP/MS | VB | TN |
|---|---|---|---|---|
| mg/g of Treatment (mg kg−1 of Dose Administered) | ||||
| 500 | MECt | 107.97 (53.99) | 265.07 (132.54) | 12.07 (6.04) |
| 100 | FCt-2 | 342.11 (34.21) | 361.76 (36.18) | 3.09 (0.31) |
| FCt-3 | 42.43 (4.24) | 153.93 (15.39) | 11.42 (1.14) | |
| FCt-4 | 30.18 (3.02) | 49.24 (4.92) | 3.74 (0.37) | |
| Dose (mg/kg) | Group | Total Area Lesion (mm2) | % of Lesion Area | Ulcer Index | Inhibition (%) |
|---|---|---|---|---|---|
| BASAL | |||||
| VEH | 47.36 ± 9.80 | 23.012 ± 8.34 | 16.5 ± 1.6 | NA | |
| 15 | IMI | 0.36 ± 0.14 * | 0.110 ± 0.05 * | 1.5 ± 0.67 | 99.2 |
| 0.05 | MISO | 0.06 ± 0.01 * | 0.017 ± 0.003 * | 0.33 ± 0.2 | 99.8 |
| 500 | MECt | 1.10 ± 0.47 * | 0.385 ± 0.172 * | 4.1 ± 1.7 | 97.6 |
| 100 | FCt-2 | 3.33 ± 1.38 * | 1.854 ± 0.988 * | 3.33 ± 1.2 | 92.9 |
| FCt-3 | 0.27 ±0.08 * | 0.122 ± 0.047 * | 2.8 ± 0.6 | 99.4 | |
| FCt-4 | 18.11 ± 4.90 * | 10.916 ± 4.36 * | 12.6 ± 2.1 | 61.7 | |
| 1 | GP/MS | 0.10 ± 0.04 * | 0.026 ± 0.011 * | 2.1 ± 0.4 | 99.7 |
| VB | 0.05 ± 0.02 * | 0.021 ± 0.002 * | 0.66 ± 0.3 | 99.8 | |
| TN | 0.32 ± 0.12 * | 0.089 ± 0.033 * | 0.33 ± 0.2 | 99.3 | |
| 2 | GP/MS | 0.10 ± 0.02 * | 0.026 ± 0.008 * | 0.83 ± 0.3 | 99.7 |
| VB | 0.15 ± 0.03 * | 0.045 ± 0.011 * | 1.16 ± 0.4 | 99.6 | |
| TN | 0.48 ± 0.06 * | 0.137 ± 0.015 * | 1.0 ± 0.2 | 98.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rodríguez, R.; Herrera-Ruiz, M.; Trejo-Tapia, G.; Domínguez-Mendoza, B.E.; González-Cortazar, M.; Zamilpa, A. In Vivo Gastroprotective and Antidepressant Effects of Iridoids, Verbascoside and Tenuifloroside from Castilleja tenuiflora Benth. Molecules 2019, 24, 1292. https://doi.org/10.3390/molecules24071292
López-Rodríguez R, Herrera-Ruiz M, Trejo-Tapia G, Domínguez-Mendoza BE, González-Cortazar M, Zamilpa A. In Vivo Gastroprotective and Antidepressant Effects of Iridoids, Verbascoside and Tenuifloroside from Castilleja tenuiflora Benth. Molecules. 2019; 24(7):1292. https://doi.org/10.3390/molecules24071292
Chicago/Turabian StyleLópez-Rodríguez, Ricardo, Maribel Herrera-Ruiz, Gabriela Trejo-Tapia, Blanca Eda Domínguez-Mendoza, Manasés González-Cortazar, and Alejandro Zamilpa. 2019. "In Vivo Gastroprotective and Antidepressant Effects of Iridoids, Verbascoside and Tenuifloroside from Castilleja tenuiflora Benth" Molecules 24, no. 7: 1292. https://doi.org/10.3390/molecules24071292
APA StyleLópez-Rodríguez, R., Herrera-Ruiz, M., Trejo-Tapia, G., Domínguez-Mendoza, B. E., González-Cortazar, M., & Zamilpa, A. (2019). In Vivo Gastroprotective and Antidepressant Effects of Iridoids, Verbascoside and Tenuifloroside from Castilleja tenuiflora Benth. Molecules, 24(7), 1292. https://doi.org/10.3390/molecules24071292