Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
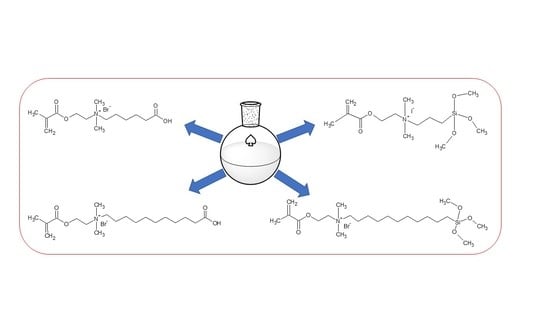
General Procedure for the Synthesis of Quaternary Ammonium (QA) Monomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Jones, R.A. Quaternary Ammonium Salts: Their Use in Phase-Transfer Catalysis; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Lygo, B.; Andrews, B.I. Asymmetric Phase-Transfer Catalysis Utilizing Chiral Quaternary Ammonium Salts: Asymmetric Alkylation of Glycine Imines. Acc. Chem. Res. 2004, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, E.J. Catalytic Enantioselective Fluorination of β-Keto Esters by Phase-Transfer Catalysis Using Chiral Quaternary Ammonium Salts. Org. Lett. 2002, 4, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Hoogerheide, J.C. The Germicidal Properties of Certain Quarternary Ammonium Salts With Special Reference to Cetyl-Trimethyl-Ammonium Bromide. J. Bacteriol. 1945, 49, 277–289. [Google Scholar] [PubMed]
- Saurino, V.R. Germicidal Use of Compositions Containing Certain Quaternary Ammonium Compounds. U.S. Patent 4,321,277, 23 March 1982. [Google Scholar]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic Biocides with Pendant Active Groups: Molecular Weight Dependence of Antibacterial Activity. Antimicrob. Agents Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. Molecular Weight Dependence of Antibacterial Activity in Cationic Disinfectants. J. Bioact. Compat. Polym. 1990, 5, 31–41. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Nonaka, T.; Hua, L.; Ogata, T.; Kurihara, S. Synthesis of water-soluble thermosensitive polymers having phosphonium groups from methacryloyloxyethyl trialkyl phosphonium chlorides–N-isopropylacrylamide copolymers and their functions. J. Appl. Polym. Sci. 2003, 87, 386–393. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary Ammonium Functionalized Poly(Propylene Imine) Dendrimers as Effective Antimicrobials: Structure-Activity Studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Bienek, D.R.; Frukhtbeyn, S.A.; Giuseppetti, A.A.; Okeke, U.C.; Skrtic, D. Antimicrobial Monomers for Polymeric Dental Restoratives: Cytotoxicity and Physicochemical Properties. J. Funct. Biomater. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.; Frukhtbeyn, S.; Giuseppetti, A.; Okeke, U.; Pires, R.; Antonucci, J.; Skrtic, D. Ionic Dimethacrylates for Antimicrobial and Remineralizing Dental Composites. Ann. Dent. Oral Disord. 2018, 2. [Google Scholar]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.C. A New Dental Cement. Br. Dent. J. 1968, 124, 381–384. [Google Scholar]
- Nicholson, J.; Czarnecka, B. Materials for the Direct Restoration of Teeth; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore Memorial Lecture. Adhesion to Enamel and Dentin: Current Status and Future Challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.K.; McDonough, W.G. Chemistry of Silanes: Interfaces in Dental Polymers and Composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541–558. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.-X.; Xu, H.H. Effects of Quaternary Ammonium Chain Length on the Antibacterial and Remineralizing Effects of a Calcium Phosphate Nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef]
- Shalaby, S.W.; Salz, U. Polymers for Dental and Orthopedic Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Menschutkin, N. Über Die Affinitätskoeffizienten Der Alkylhaloide Und Der Amine. Z. Für Phys. Chem. 1890, 6, 41–57. [Google Scholar]
- Menschutkin, N. Beiträge Zur Kenntnis Der Affinitätskoeffizienten Der Alkylhaloide Und Der Organischen Amine. Z. Für Phys. Chem. 1890, 5, 589–600. [Google Scholar] [CrossRef]
- Wedekind, E.; Wedekind, O.; Paschke, F. Abhängigkeit Der Racemisationsgeschwindigkeit Optischaktiver Ammoniumsalze von Der Natur Der Anionen. Berichte Dtsch. Chem. Ges. 1908, 41, 1029–1035. [Google Scholar] [CrossRef]
- Abboud, J.L.M.; Notario, R.; Bertran, J.; Sola, M. One Century of Physical Organic Chemistry: The Menshutkin Reaction. In Progress in Physical Organic Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1993; Volume 19, pp. 1–182. [Google Scholar]
- Acevedo, O.; Jorgensen, W.L. Exploring Solvent Effects upon the Menshutkin Reaction Using a Polarizable Force Field. J. Phys. Chem. B 2010, 114, 8425–8430. [Google Scholar] [CrossRef] [PubMed]
- Culler, S.R.; Naviroj, S.; Ishida, H.; Koenig, J.L. Analytical and Spectroscopic Investigation of the Interaction of CO2 with Amine Functional Silane Coupling Agents on Glass Fibers. J. Colloid Interf. Sci. 1983, 96, 69–79. [Google Scholar] [CrossRef]
- Peña-Alonso, R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the Hydrolysis and Condensation of γ-Aminopropyltriethoxysilane by FT-IR Spectroscopy. J. Mater. Sci. 2007, 42, 595–603. [Google Scholar] [CrossRef]
- White, L.D.; Tripp, C.P. Reaction of (3-Aminopropyl)Dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interf. Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Trujillo-Lemon, M.; Ge, J.; Stansbury, J.W. Dental resins based on dimer acid dimethacrylates: A route to high conversion with low polymerization shrinkage. Compend. Contin. Educ. Dent. 2010, 31, 1–4. [Google Scholar] [PubMed]
- Stansbury, J.W. Dimethacrylate Network Formation and Polymer Property Evolution as Determined by the Selection of Monomers and Curing Conditions. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef]
- Ali, S.A.; Rasheed, A.; Wazeer, M.I.M. Synthesis and Solution Properties of a Quaternary Ammonium Polyampholyte. Polymer 1999, 40, 2439–2446. [Google Scholar] [CrossRef]
- Winstead, A.; Hart, K.; Hijji, Y.M.; Jasinski, J.P.; Butcher, R.J. 1-(5-Carboxypentyl)-2,3,3-Trimethyl-3H-Indol-1-Ium Bromide Monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, 171–172. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Position | δC | δH |
|---|---|---|
| 1 | 126.5 | 5.76, s; 6.08, s |
| 2 | 135.3 | |
| 3 | 17.8 | 1.91, s |
| 4 | 165.8 | |
| 5 | 58.0 | 4.52, m |
| 6 | 61.6 | 3.70, m |
| 7, 8 | 50.4 | 3.09, s |
| 9 | 63.6 | 3.36, m |
| 10 | 21.5 | 1.69, m |
| 11 | 25.2 | 1.28, m |
| 12 | 23.9 | 1.55, m |
| 13 | 33.2 | 2.24, t |
| 14 | 174.2 | |
| 19 | 12.04, s |
| Position | δC | δH |
|---|---|---|
| 1 | 126.5 | 5.76, s; 6.08, s |
| 2 | 135.3 | |
| 3 | 17.8 | 1.91, s |
| 4 | 165.8 | |
| 5 | 58.1 | 4.52, m |
| 6 | 61.6 | 3.70, m |
| 7, 8 | 50.4 | 3.09, s |
| 9 | 63.8 | 3.35, m |
| 10 | 21.7 | 1.67, m |
| 11–16 | 25.7, 28.37, 28.43, 28.58, 28.64, 28.67 | 1.26, m |
| 17 | 24.4 | 1.48, q |
| 18 | 33.6 | 2.19, t |
| 19 | 174.4 | |
| 20 | 11.96, s |
| Position | δC | δH |
|---|---|---|
| 1 | 126.6 | 5.77, s; 6.09, s |
| 2 | 135.3 | |
| 3 | 17.8 | 1.92, s |
| 4 | 165.8 | |
| 5 | 58.0 | 4.52, m |
| 6 | 61.7 | 3.70, m |
| 7, 8 | 50.6 | 3.09, s |
| 9 | 65.9 | 3.34, m |
| 10 | 15.6 | 1.72, m |
| 11 | 5.2 | 0.54, t |
| 12,13,14 | 50.1 | 3.51, s |
| Position | δC | δH |
|---|---|---|
| 1 | 126.5 | 5.76, s; 6.08, s |
| 2 | 135.3 | |
| 3 | 17.8 | 1.91, s |
| 4 | 165.8 | |
| 5 | 58.1 | 4.52, m |
| 6 | 61.6 | 3.70, m |
| 7, 8 | 50.4 | 3.09, s |
| 9 | 63.8 | 3.36, m |
| 10 | 21.7 | 1.67, m |
| 11–18 | 22.1, 25.7, 28.4, 28.6, 28.7, 28.8, 28.9, 32.3 | 1.25, m |
| 19 | 8.6 | 0.57, t |
| 20,21,22 | 49.9 | 3.46, s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okeke, U.C.; Snyder, C.R.; Frukhtbeyn, S.A. Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds. Molecules 2019, 24, 1464. https://doi.org/10.3390/molecules24081464
Okeke UC, Snyder CR, Frukhtbeyn SA. Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds. Molecules. 2019; 24(8):1464. https://doi.org/10.3390/molecules24081464
Chicago/Turabian StyleOkeke, Ugochukwu C., Chad R. Snyder, and Stanislav A. Frukhtbeyn. 2019. "Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds" Molecules 24, no. 8: 1464. https://doi.org/10.3390/molecules24081464
APA StyleOkeke, U. C., Snyder, C. R., & Frukhtbeyn, S. A. (2019). Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds. Molecules, 24(8), 1464. https://doi.org/10.3390/molecules24081464