Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds
Abstract
:1. Introduction
2. Results and Discussion
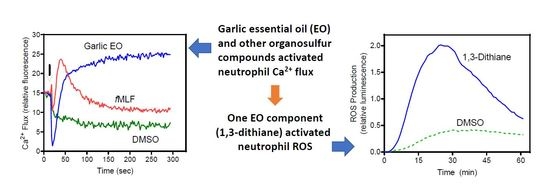
2.1. Effect of Garlic EO and Organosulfur Compounds on Neutrophil Ca2+ Flux
2.2. Effect of Garlic EO and Organosulfur Compounds on Neutrophil ROS Production
2.3. Effect of Phosphatidylinositol-3 Kinase (PI3K) Inhibitors
2.4. Effect of 1,3-Dithiane on Protein Kinase Phosphorylation
3. Materials and Methods
3.1. Screening Compounds and Garlic EO
3.2. Materials for Biological Assays
3.3. Isolation of Human Neutrophils
3.4. Cell Culture
3.5. Ca2+ Mobilization Assay
3.6. ROS Production Assay
3.7. Protein Kinase Array
3.8. ERK1/2 Enzyme-Linked Immunosorbent Assay (ELISA)
3.9. Analysis of 1,3-Dithiane Biotransformation
3.10. Molecular Modeling
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADME | absorption, distribution, metabolism, and excretion |
| AITC | allyl isothiocyanate |
| CL | chemiluminescent |
| CREB | cAMP response element binding |
| DFT | density functional theory |
| DMSO | dimethyl sulfoxide |
| EI | electron impact |
| ELISA | enzyme-linked immunosorbent assay |
| EO | essential oil |
| ERK1/2 | extracellular signal–regulated kinase 1/2 |
| GC-MS | gas chromatography–mass spectrometry |
| GPCR | G-protein-coupled receptor |
| GSK-3α/β | glycogen synthase kinase 3 α/β |
| HOMO | highest occupied molecular orbital |
| hsp27 | heat shock protein 27 |
| JNK | c-Jun N-terminal kinases |
| LUMO | lowest unoccupied molecular orbital |
| MAPK | mitogen activated protein kinase |
| MKK | MAP kinase kinase |
| MSK | mitogen- and stress-activated kinase |
| mTOR | mammalian target of rapamycin |
| NADPH | reduced nicotinamide adenine dinucleotide phosphate |
| NO | nitric oxide |
| p70S6K1 | p70 S6 kinase 1 |
| PIP3 | phosphatidylinositol (3,4,5)-trisphosphate |
| PMA | phorbol-12-myristate-13-acetate |
| PI3K | phosphatidylinositol-3 kinase |
| PIP3 | phosphatidylinositol (3,4,5)-trisphosphate |
| ROS | reactive oxygen species |
| RSK | p90 ribosomal S6 kinase |
| SOD | superoxide dismutase |
| TRP | transient receptor potential |
References
- Petropoulos, S.; Di Gioia, F.; Ntatsi, G. Vegetable organosulfur compounds and their health promoting effects. Curr. Pharm. Des. 2017, 23, 2850–2875. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef]
- Cao, H.-X.; Zhu, K.-X.; Fan, J.-G.; Qiao, L. Garlic-derived allyl sulfides in cancer therapy. Anti-Cancer Agents Med. Chem. 2014, 14, 793–799. [Google Scholar] [CrossRef]
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.-X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Kovačević, D.B. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water-based delivery systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.; Kaschula, C.H. The Immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-Cancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2018, 24, 119. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)—A review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic role of functional components in alliums for preventive chronic disease in human being. Evidence-Based Complement. Altern. Med. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Özek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Özek, T.; Başer, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Mohammadi, M.; Habibi, Z. Disulfides in the volatile oil of Ferula behboudiana Rech. f. & Esfand. Nat. Prod. Res. 2011, 25, 1629–1634. [Google Scholar] [PubMed]
- Yu, T.H.; Wu, C.M.; Liou, Y.C. Volatile compounds from garlic. J. Agric. Food Chem. 1989, 37, 725–730. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Ban, J.O.; Park, K.-R.; Kil Lee, C.; Jeong, H.-S.; Han, S.B.; Hong, J.T. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol. Ther. 2014, 142, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-C.; Kuo, C.-H.; Juang, J.-Y.; Liu, C.-H.; Hsu, L.; Liu, C.-T. Effects of garlic oil on the migration of neutrophil-like cell studied by using a chemotactic gradient Labchip. J. Biomed. Biotechnol. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Lee, S.-H.; Chen, K.-M.; Lii, C.-K.; Liu, C.-T. Effect of garlic oil on neutrophil infiltration in the small intestine of endotoxin-injected rats and its association with levels of soluble and cellular adhesion molecules. J. Agric. Food Chem. 2011, 59, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, C.; Arbach, M.; Meyer, D.; Hamilton, C.; Lall, N. Diallyl polysulfides from Allium sativum as immunomodulators, hepatoprotectors, and antimycobacterial agents. J. Med. Food 2017, 20, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, R.; Frass, M.; Gmeiner, B.; Kaye, A.D.; Frost, E.A.M. Effects of garlic extract (Allium sativum) on neutrophil migration at the cellular level. Heart Dis. 2001, 3, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Miltojević, A.B.; Stojković, M.B.; Blagojević, P.D. New volatile sulfur-containing compounds from wild garlic (Allium ursinum L., Liliaceae). Food Res. Int. 2015, 78, 1–10. [Google Scholar] [CrossRef]
- Nohara, T.; Fujiwara, Y.; Ikeda, T.; Murakami, K.; Ono, M.; Nakano, D.; Kinjo, J. Cyclic Sulfoxides garlicnins B2, B3, B4, C2, and C3 from Allium sativum. Chem. Pharm. 2013, 61, 695–699. [Google Scholar] [CrossRef]
- Nohara, T.; Fujiwara, Y.; Komota, Y.; Kondo, Y.; Saku, T.; Yamaguchi, K.; Komohara, Y.; Takeya, M. Cyclic sulfoxides - garlicnins K1, K2, and H1 - extracted from Allium sativum. Chem. Pharm. 2015, 63, 117–121. [Google Scholar] [CrossRef]
- Ono, M.; Fujiwara, Y.; Ikeda, T.; Pan, C.; El-Aasr, M.; Lee, J.H.; Nakano, D.; Kinjo, J.; Nohara, T. Atypical cyclic sulfides, garlicnins G, I, and J, extracted from Alium sativum. Chem. Pharm. Bull. (Tokyo) 2017, 65, 102–106. [Google Scholar] [CrossRef]
- Navegantes, K.C.; Gomes, R.D.S.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 328. [Google Scholar] [CrossRef]
- Teng, T.-S.; Ji, A.-L.; Ji, X.-Y.; Li, Y.-Z. Neutrophils and immunity: From bactericidal action to being conquered. J. Immunol. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Skovbakke, S.L.; Holdfeldt, A.; Forsman, H.; Bylund, J.; Franzyk, H. The role of formyl peptide receptors for immunomodulatory activities of antimicrobial peptides and peptidomimetics. Curr. Pharm. Des. 2018, 24, 1100–1120. [Google Scholar] [CrossRef]
- Favarin, D.C.; De Oliveira, J.R.; De Oliveira, C.J.F.; Rogerio, A.D.P. Potential effects of medicinal plants and secondary metabolites on acute lung injury. BioMed Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Khlebnikov, A.I.; Kirpotina, L.N.; Quinn, M.T. Antagonism of human formyl peptide receptor 1 with natural compounds and their synthetic derivatives. Int. Immunopharmacol. 2016, 37, 43–58. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, V.; Roshan, R.; Sharma, A.; Rembhotkar, G.W.; Ghosh, B. Piperine inhibits TNF-α induced adhesion of neutrophils to endothelial monolayer through suppression of NF-κB and I-κB kinase activation. Eur. J. Pharmacol. 2007, 575, 177–186. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Ozek, T.; Baser, K.H.C.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.C.; Kovrizhina, A.R.; et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef]
- Lee, S.-H.; Liu, Y.-T.; Chen, K.-M.; Lii, C.-K.; Liu, C.-T. Effect of garlic sulfur compounds on neutrophil infiltration and damage to the intestinal mucosa by endotoxin in rats. Food Chem. Toxicol. 2012, 50, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Hong, J.; Jeon, C.M.; Shin, N.R.; Kwon, O.K.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the NRF-2/HO-1 pathway and NF-κB suppression. Food Chem. Toxicol. 2013, 62, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-L.; Chen, H.-W.; Wang, R.-Y.; Lei, Y.-P.; Sheen, L.-Y.; Lii, C.-K. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-κB activation in RAW 264.7 macrophages. J. Agric. Food Chem. 2006, 54, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-P.; Chen, Y.-H. Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition 2005, 21, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ippoushi, K.; Itou, H.; Azuma, K.; Higashio, H. Effect of naturally occurring organosulfur compounds on nitric oxide production in lipopolysaccharide-activated macrophages. Life Sci. 2002, 71, 411–419. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Liu, K.-L.; Lin, C.-Y.; Chen, H.-W.; Lii, C.-K. Structure and function relationship study of Allium organosulfur compounds on upregulating the pi class of glutathione S-transferase expression. J. Agric. Food Chem. 2011, 59, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Na Zhao, N.; Luan, X.B.; Shi, W.P.; Liu, Q.Z.; Zhang, H.; Zhou, C. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J. Econ. Èntomol. 2013, 106, 1349–1354. [Google Scholar] [CrossRef]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, S.-Q.; Zhang, J.; Huang, G.-Y.; Chen, L.-Y.; Zhao, F.-Y. Chemical composition, antimicrobial property and microencapsulation of mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem. 2014, 165, 560–568. [Google Scholar] [CrossRef]
- Fratianni, F.; Riccardi, R.; Spigno, P.; Ombra, M.N.; Cozzolino, A.; Tremonte, P.; Coppola, R.; Nazzaro, F. Biochemical characterization and antimicrobial and antifungal activity of two endemic varieties of garlic (Allium sativum L.) of the Campania region, Southern Italy. J. Med. Food 2016, 19, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Choi, E.H.; Lee, K.-G.; Chun, H.S. Alleviation of aflatoxin B1-induced oxidative stress in HepG2 cells by volatile extract from Allii Fistulosi Bulbus. Life Sci. 2005, 77, 2896–2910. [Google Scholar] [CrossRef]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.; Quinn, M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol. Pharmacol. 2007, 71, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Al-Zereini, W.; Fotso Fondja Yao, C.B.; Laatsch, H.; Anke, H. Aqabamycins A-G: Novel nitro maleimides from a marine Vibrio species. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 2010, 63, 297–301. [Google Scholar] [CrossRef]
- Lee, H.; Bian, S.S.; Chen, Y.L. Genotoxicity of 1,3-dithiane and 1,4-dithiane in the CHO/SCE assay and the Salmonella/microsomal test. Res. Toxicol. 1994, 321, 213–218. [Google Scholar]
- Zambianchi, F.; Raimondi, S.; Pasta, P.; Carrea, G.; Gaggero, N.; Woodley, J.A. Comparison of cyclohexanone monooxygenase as an isolated enzyme and whole cell biocatalyst for the enantio selective oxidation of 1,3-dithiane. J. Mol. Catal. B-Enzym. 2004, 31, 165–171. [Google Scholar] [CrossRef]
- Condliffe, A.M.; Davidson, K.; Anderson, K.E.; Ellson, C.D.; Crabbe, T.; Okkenhaug, K.; Vanhaesebroeck, B.; Turner, M.; Webb, L.; Wymann, M.P.; et al. Sequential activation of class 1A and class 1B PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 2005, 106, 1432–1440. [Google Scholar] [CrossRef]
- Boyle, K.B.; Gyori, D.; Sindrilaru, A.; Scharffetter-Kochanek, K.; Taylor, P.R.; Mocsai, A.; Stephens, L.R.; Hawkins, P.T. Class 1A phosphoinositide 3-kinase β and δ regulate neutrophil oxidase activation in response to Aspergillus fumigatus hyphae. J. Immunol. 2011, 186, 2978–2989. [Google Scholar] [CrossRef]
- Jackson, S.P.; Schoenwaelder, S.M.; Goncalves, I.; Nesbitt, W.S.; Yap, C.L.; E Wright, C.; Kenche, V.; E Anderson, K.; Dopheide, S.M.; Yuan, Y.; et al. PI 3-kinase p110β: A new target for antithrombotic therapy. Nat. Med. 2005, 11, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Camps, M.; Rückle, T.; Ji, H.; Ardissone, V.; Rintelen, F.; Shaw, J.; Ferrandi, C.; Chabert, C.; Gillieron, C.; Françon, B.; et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 2005, 11, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.; Flanagan, J.U.; Kolekar, S.; Buchanan, C.; Kendall, J.D.; Rewcastle, G.W.; Denny, W.A.; Singh, R.; Dickson, J.; Baguley, B.C.; et al. A drug targeting only p110α can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. Biochem. J. 2011, 438, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, L.E.; Sun, S.; Li, H.; Vary, T.C.; Korchak, H.M. Regulation of TNF-induced oxygen radical production in human neutrophils: Role of δ-PKC. J. Leukoc. Biol. 2010, 87, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hazan-Halevy, I.; Levy, T.; Wolak, T.; Lubarsky, I.; Lévy, R.; Paran, E. Stimulation of NADPH oxidase by angiotensin II in human neutrophils is mediated by ERK, p38 MAP-kinase and cytosolic phospholipase A2. J. Hypertens. 2005, 23, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hui, W.; Fernandes, M.J.; Poubelle, P.E.; Bourgoin, S.G. Lysophosphatidic acid-induced IL-8 secretion involves MSK1 and MSK2 mediated activation of CREB1 in human fibroblast-like synoviocytes. Biochem. Pharmacol. 2014, 90, 62–72. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef]
- Kalff, H.T.; Havinga, E. Conformation of non-aromatic ring compounds. 20. Dipole moments and NMR spectra of some 2-substituted 1,3-dithianes. Recl. Trav. Chim. Pays-Bas 1966, 85, 467–484. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, Y.; Shirato, K.; Abe, I.; Kobayashi, A.; Mitsuhashi, R.; Shiono, C.; Sato, S.; Tachiyashiki, K.; Imaizumi, K. Diallyl disulfide reduced dose-dependently the number of lymphocyte subsets and monocytes in rats. J. Nutr. Sci. Vitaminol. 2012, 58, 292–296. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 2007, 110, 1083–1095. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Huang, C.F.; Tseng, Y.T.; Kuo, S.Y. Diallyl disulfide induces Ca2+ mobilization in human colon cancer cell line SW480. Arch. Toxicol. 2012, 86, 231–238. [Google Scholar] [CrossRef]
- Jan, C.R.; Lo, H.R.; Chen, C.Y.; Kuo, S.Y. Effect of allyl sulfides from garlic essential oil on intracellular Ca2+ levels in renal tubular cells. J. Nat. Prod. 2012, 75, 2101–2107. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.-E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Acad. Sci. 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Boil. 2005, 15, 929–934. [Google Scholar] [CrossRef]
- Koizumi, K.; Iwasaki, Y.; Narukawa, M.; Iitsuka, Y.; Fukao, T.; Seki, T.; Ariga, T.; Watanabe, T. Diallyl sulfides in garlic activate both TRPA1 and TRPV1. Biochem. Biophys. Commun. 2009, 382, 545–548. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Chianese, G.; Appendino, G.B.; Di Marzo, V.; De Petrocellis, L.; Ghannadi, A.; Taghvayi, R.; Fattahian, K.; Soltani, R.; Taglialatela-Scafati, O. Some like it pungent and vile. TRPA1 as a molecular target for the malodorous vinyl disulfides from asafoetida. Fitoterapia 2013, 90, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.A.; Alpizar, Y.; Boonen, B.; Sanchez, A.; Everaerts, W.; Segal, A.; Xue, F.; Janssens, A.; Owsianik, G.; Nilius, B.; et al. Mechanisms of transient receptor potential vanilloid 1 activation and sensitization by allyl isothiocyanate. Mol. Pharmacol. 2013, 84, 325–334. [Google Scholar] [CrossRef]
- Yassaka, R.T.; Inagaki, H.; Fujino, T.; Nakatani, K.; Kubo, T. Enhanced activation of the transient receptor potential channel TRPA1 by ajoene, an allicin derivative. Neurosci. Res. 2010, 66, 99–105. [Google Scholar] [CrossRef]
- Heiner, I.; Eisfeld, J.; Lückhoff, A. Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 2003, 33, 533–540. [Google Scholar] [CrossRef]
- Immler, R.; Simon, S.I.; Sperandio, M. Calcium signalling and related ion channels in neutrophil recruitment and function. Eur. J. Clin. Investig. 2018, 48, e12964. [Google Scholar] [CrossRef]
- Daiber, A.; August, M.; Baldus, S.; Wendt, M.; Oelze, M.; Sydow, K.; Kleschyov, A.L.; Münzel, T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Radic. Boil. Med. 2004, 36, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
Sample Availability: no available. |




| Compound Name | Chemical Structure | Plant Species | % a | References |
|---|---|---|---|---|
| Allyl methyl sulfide |  | Allium ursinum | 0.0–0.1 | [20] |
| Allium sativum | tr | [13] | ||
| Dipropyl sulfide |  | Allium sativum | n.d. | [37] |
| Allyl propyl sulfide |  | Allium ursinum | tr | [20] |
| Allium sativum | 0.0–0.1 | [38] | ||
| Diallyl sulfide |  | Allium sativum | 1.6–9.5 | [38,39,40] |
| Allium ursinum | 0.1–0.3 | [20] | ||
| Dimethyl disulfide |  | Allium sativum | 0.4–1.4 | [38] |
| Allium ursinum | 0.7–2.3 | [20] | ||
| Methyl propyl disulfide |  | Allium sativum | tr | [13] |
| Dipropyl disulfide |  | Allium porrum | 29.8 | [40] |
| Allium ursinum | 0.0–0.3 | [20] | ||
| Allyl methyl disulfide |  | Allium sativum | 4.4–8.3 | [38] |
| Allium ursinum | 1.1–18.9 | [20] | ||
| Allyl propyl disulfide |  | Allium sativum | 3.1 | [39] |
| Diallyl disulfide |  | Allium ursinum | 9.9–20.7 | [20] |
| Allium sativum | 20.8–29.1 | [38,39,40] | ||
| Dimethyl trisulfide |  | Allium sativum | 1.3–2.9 | [38] |
| Allium ursinum | 1.1–7.5 | [20] | ||
| Dipropyl trisulfide |  | Allium ursinum | tr | [20] |
| Diallyl trisulfide |  | Allium sativum | 16.8–50.4 | [38,39,40] |
| Allium ursinum | 5.2–19.6 | [20] | ||
| Allyl isothiocyanate (AITC) |  | Sinapis alba (mustard seed) | 71.1 | [41] |
| Allicin |  | Allium sativum | (3 mg/g) b | [42] |
| 2,5-Dimethylthiophene |  | Allium fistulosum | tr | [43] |
| 1,3-Dithiane |  | Allium sativum | 2.1 | [13,39] |
| Compound Common Name | Ca2+ Flux | Spontaneous CL | Stimulated CL | |
|---|---|---|---|---|
| Activation EC50 (µM) | Inhibition IC50 (µM) | Inhibition IC50 (µM) | ||
| Allyl methyl sulfide | N.A. | N.A. | N.A. | N.A. |
| Dipropyl sulfide | N.A. | N.A. | N.A. | N.A. |
| Allyl propyl sulfide | N.A. | N.A. | N.A. | N.A. |
| Diallyl sulfide | N.A. | N.A. | N.A. | N.A. |
| Dimethyl disulfide | N.A. | N.A. | N.A. | N.A. |
| Methyl propyl disulfide | N.A. | 30.2 ± 4.6 | N.A. | N.A. |
| Dipropyl disulfide | 13.1 ± 3.4 | 29.1 ± 4.8 | N.A. | N.A. |
| Allyl methyl disulfide | N.A. | N.A. | N.A. | N.A. |
| Allyl propyl disulfide | 22.5 ± 6.2 | 18.6 ± 4.1 | N.A. | N.A. |
| Diallyl disulfide | 9.8 ± 2.1 | 22.1 ± 3.7 | N.A. | N.A. |
| Dimethyl trisulfide | N.A. | N.A. | N.A. | N.A. |
| Dipropyl trisulfide | N.A. | N.A. | N.A. | N.A. |
| Diallyl trisulfide | N.A. | N.A. | 17.7 ± 3.3 | 39.0 ± 5.2 |
| Allyl isothiocyanate (AITC) | 7.9 ± 1.8 | 20.8 ± 4.3 | N.A. | N.A. |
| Allicin | N.A. | 30.7 ± 5.1 | 1.5 ± 0.3 | 4.4 ± 0.6 |
| Ajoene | N.A. | N.A. | 5.5 ± 1.4 | 22.1 ± 4.1 |
| Alliin | N.A. | N.A. | N.A. | 17.5 ± 2.8 |
| N-acetyl-S-allyl-L-cysteine | N.A. | N.A. | N.A. | 9.5 ± 2.1 |
| S-allyl-L-cysteine | N.A. | N.A. | N.A. | 10.9 ± 0.9 |
| 2,5-Dimethylthiophene | N.A. | N.A. | N.A. | N.A. |
| Cyclopentanethiol | N.A. | N.A. | N.A. | N.A. |
| 1,3-Dithiane | N.A. | N.A. | * | |
| (µg/mL) | ||||
| Garlic EO | 34.9 ± 2.8 | 14.7 ± 2.4 | 5.5 ± 1.2 | 3.7 ± 0.8 |
| Properties | 1,3-Dithiane | 1,4-Dithiane |
|---|---|---|
| 2D Structure |  |  |
| Formula | C4H8S2 | |
| Molecular weight | 120.24 g/mol | |
| Num. heavy atoms | 6 | |
| Fraction Csp3 | 1.00 | |
| Num. rotatable bonds | 0 | |
| Num. H-bond acceptors | 0 | |
| Num. H-bond donors | 0 | |
| Molar refractivity | 34.41 | |
| Topological polar surface area | 50.60 Ų | |
| Lipophilicity (consensus Log Po/w) | 1.86 | 1.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Balasubramanian, N.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds. Molecules 2019, 24, 1809. https://doi.org/10.3390/molecules24091809
Schepetkin IA, Kirpotina LN, Khlebnikov AI, Balasubramanian N, Quinn MT. Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds. Molecules. 2019; 24(9):1809. https://doi.org/10.3390/molecules24091809
Chicago/Turabian StyleSchepetkin, Igor A., Liliya N. Kirpotina, Andrei I. Khlebnikov, Narayanaganesh Balasubramanian, and Mark T. Quinn. 2019. "Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds" Molecules 24, no. 9: 1809. https://doi.org/10.3390/molecules24091809
APA StyleSchepetkin, I. A., Kirpotina, L. N., Khlebnikov, A. I., Balasubramanian, N., & Quinn, M. T. (2019). Neutrophil Immunomodulatory Activity of Natural Organosulfur Compounds. Molecules, 24(9), 1809. https://doi.org/10.3390/molecules24091809