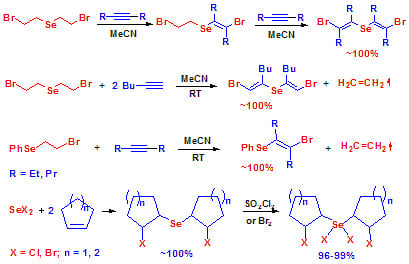
Remarkable Alkene-to-Alkene and Alkene-to-Alkyne Transfer Reactions of Selenium Dibromide and PhSeBr. Stereoselective Addition of Selenium Dihalides to Cycloalkenes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Compounds 7a,b–10a,b
3.3. Synthesis of Compounds 17–26
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Gladyshev, V.N.; Hatfield, D.L. Selenocysteine-Containing Proteins in Mammals. J. Biomed. Sci. 1999, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef]
- Potapov, V.A. Organic diselenides, ditellurides, polyselenides and polytellurides. Synthesis and reactions. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 765–843. [Google Scholar]
- Organoselenium Chemistry: Synthesis and Reactions; Wirth, T. (Ed.) Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Organoselenium Chemistry: Between Synthesis and Biochemistry; Santi, C. (Ed.) Bentham Science Publishers: Sharjah, UAE, 2014. [Google Scholar]
- Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Woollins, J.D., Laitinen, R.S., Eds.; Springer: Heidelberg, Germany, 2011. [Google Scholar]
- Wirth, T. Organoselenium Chemistry—Modern Developments in Organic Synthesis, Topics in Current Chemistry; 208; Springer: Heidelberg, Germany, 2000. [Google Scholar]
- Nicolaou, K.C.; Petasi, N.A. Selenium in Natural Products Synthesis; CIS: Philadelphia, PA, USA, 1984. [Google Scholar]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon: Oxford, UK, 1986. [Google Scholar]
- Back, T.G. Organoselenium Chemistry: A Practical Approach; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Potapov, V.A.; Trofimov, B.A. 1-(Organosulfanyl)-, 1-(organoselanyl)-, and 1-(organotellanyl)alk-1-ynes. Sci. Synth. 2005, 24, 957–1005. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Belozerova, O.V.; Albanov, A.I.; Yarosh, O.G.; Voronkov, M.G. Synthesis of 3,6-dihalo-4,4-dimethyl-1,4-selenasilafulvenes. Chem. Heterocycl. Compd. 2003, 39, 549–550. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Maaninen, A.; Chivers, T.; Parvez, M.; Pietikäinen, J.; Laitinen, R.S. Syntheses of THF Solutions of SeX2 (X = Cl, Br) and a New Route to Selenium Sulfides SenS8-n (n = 1–5): X-ray Crystal Structures of SeCl2(tht)2 and SeCl2·tmtu. Inorg. Chem. 1999, 38, 4093–4097. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity. Synthesis 2014, 46, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Sarbu, L.G.; Hopf, H.; Jones, P.G.; Birsa, L.M. Selenium halide-induced bridge formation in [2.2]paracyclophanes. Beilstein J. Org. Chem. 2014, 10, 2550–2555. [Google Scholar] [CrossRef] [Green Version]
- Arsenyan, P. A simple method for the preparation of selenopheno[3,2-b] and [2,3-b]thiophenes. Tetrahedron Lett. 2014, 55, 2527–2529. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Regioselective Synthesis of Bis[(2,3-dihydro-1-benzofuran-2-yl)methyl]selenide. Russ. J. Org. Chem. 2014, 50, 1702–1703. [Google Scholar]
- Arsenyan, P.; Petrenko, A.; Belyakov, S. Improved conditions for the synthesis and transformations of aminomethyl selenophenothiophenes. Tetrahedron 2015, 71, 2226–2233. [Google Scholar] [CrossRef]
- Musalov, M.V.; Musalova, M.V.; Potapov, V.A.; Albanov, A.I.; Amosova, S.V. Methoxyselenation of Cyclopentene with Selenium Dibromide. Russ. J. Org. Chem. 2015, 51, 1662–1663. [Google Scholar] [CrossRef]
- Musalov, M.V.; Musalova, M.V.; Potapov, V.A.; Amosova, S.V. Effective Synthesis of 2,2′-[Selanediylbis(cycloalkyl)] Diacetates. Russ. J. Org. Chem. 2016, 52, 1207–1208. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Kurkutov, E.O.; Musalova, M.V.; Albanov, A.I.; Amosova, S.V. Synthesis of New Functionalized Organoselenium Compounds by Heterocyclization of Selenium Dihalides with Pent-4-en-1-ol. Russ. J. Org. Chem. 2016, 52, 339–342. [Google Scholar] [CrossRef]
- Volkova, Y.M.; Makarov, A.Y.; Zikirin, S.B.; Genaev, A.M.; Bagryanskaya, I.Y.; Zibarev, A.V. 3,1,2,4-Benzothiaselenadiazine and related heterocycles. Mendeleev Commun. 2017, 27, 19–22. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. The reaction of selenium dichloride with divinyl sulfide. J. Organomet. Chem. 2009, 694, 3369–3372. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Addition of selenium dibromide to divinyl sulfide: Spontaneous rearrangement of 2,6-dibromo-1,4-thiaselenane to 5-bromo-2-bromomethyl-1,3-thiaselenolane. Tetrahedron Lett. 2009, 50, 306–308. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Synthesis of 2,6-Dichloro-1,4-thiaselenane from Divinyl Sulfide and Selenium Dichloride. Russ. J. Org. Chem. 2009, 45, 1271–1272. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I. Reactions of selenium dichloride and dibromide with divinyl selenide: Synthesis of novel selenium heterocycles and rearrangement of 2,6-dihalo-1,4-diselenanes. Tetrahedron Lett. 2010, 51, 89–92. [Google Scholar] [CrossRef]
- Potapov, V.A.; Shagun, V.A.; Penzik, M.V.; Amosova, S.V. Quantum chemical studies of the reaction of selenium dichloride with divinyl sulfide and comparison with experimental results. J. Organomet. Chem. 2010, 695, 1603–1609. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Expedient Procedure for Preparation of 2-Chloromethyl-1,3-diselenol. Russ. J. Gen. Chem. 2009, 79, 1225. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Reaction of Selenium Dichloride with Divinyl Selenide. Russ. J. Org. Chem. 2008, 44, 1556–1557. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Synthesis of 4-Bromo-2-bromomethyl-1,3-diselenolane from Selenium Dibromide and Divinyl Selenide. Russ. J. Gen. Chem. 2008, 78, 1990–1991. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with divinyl sulfone: Synthesis of novel four- and five-membered selenium heterocycles. Tetrahedron Lett. 2010, 51, 5258. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Albanov, A.I.; Amosova, S.V. Regio- and Stereoselective Addition of Selenium Dibromide to Divinyl Sulfone. Russ. J. Org. Chem. 2008, 44, 1547–1548. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Amosova, S.V. Synthesis of a New Four-Membered Heterocycle by Reaction of Selenium Dichloride with Divinyl Sulfone. Russ. J. Org. Chem. 2010, 46, 1099–1100. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Amosova, S.V. Stereoselective Synthesis of 5-Bromo-2-bromomethyl-1,3-thiaselenolane 1,1-Dioxide by Addition of Selenium Dibromide to Divinyl Sulfone. Russ. J. Gen. Chem. 2010, 80, 1220–1221. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B.; Potapov, V.A.; Penzik, M.V.; Amosova, S.V. Conformational analysis and diastereotopic assignments in the series of selenium-containing heterocycles by means of 77Se-1H spin-spin coupling constants: A combined theoretical and experimental study. Magn. Reson. Chem. 2011, 49, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and their complexes with selenium dihalides: Synthesis and structural characterization. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Abramova, E.V.; Sterkhova, I.V.; Molokeev, M.S.; Potapov, V.A.; Amosova, S.V. First coordination compounds of SeBr2 with selenium ligands: X-ray structural determination. Mendeleev Commun. 2016, 26, 532–534. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Reaction of selenium dichloride with allyl phenyl ether. Russ. J. Org. Chem. 2011, 47, 948–949. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Annulation of phenyl propargyl ether with selenium dichloride. Russ. Chem. Bull. Int. Ed. 2010, 60, 767–768. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Kurkutov, E.O.; Musalova, M.V.; Khabibulina, A.G.; Amosova, S.V. Regioselective syntheses of bis-(2-haloalkyl) selenides and dihalo[bis-(2-haloalkyl)]-λ4-selanes from selenium dihalides and 1-alkenes, and the methoxyselenenylation reaction. Archivoc 2017, iii, 365–376. [Google Scholar]
- Kurkutov, E.O.; Musalov, M.V.; Potapov, V.A.; Larina, L.I.; Amosova, S.V. Rearrangements in methanolysis of bis(2-bromoalkyl)selenides. Russ. J. Org. Chem. 2016, 52, 186–191. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Stereoselective synthesis of (E,E)-bis(2-halovinyl) selenides and its derivatives based on selenium halides and acetylene. Tetrahedron 2012, 68, 10567–10572. [Google Scholar] [CrossRef]
- Amosova, S.V.; Musalov, M.V.; Martynov, A.V.; Potapov, V.A. Regio- and Stereoselective Addition of Selenium Dihalogenides to Propargyl Halogenides. Russ. J. Gen. Chem. 2011, 81, 1239–1240. [Google Scholar] [CrossRef]
- Musalov, M.V.; Martynov, A.V.; Amosova, S.V.; Potapov, V.A. Stereo- and Regioselective Reaction of Selenium Dichloride and Dibromide with Ethynyl(trimethyl)silane. Russ. J. Org. Chem. 2012, 48, 1571–1573. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Rusakov, Y.Y.; Khabibulina, A.G.; Rusakova, I.L.; Amosova, S.V. Stereoselective synthesis of E-2-halovinyl tellanes, ditellanes and selenides based on tellurium tetrahalides, selenium dihalides and internal alkynes. J. Organomet. Chem. 2018, 867, 300–305. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Reaction of selenium dichloride with trimethylpropargylsilane. Russ. Chem. Bull. Int. Ed. 2011, 60, 769–770. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Reaction of Diselenium Dichloride with Acetylene. Russ. J. Org. Chem. 2011, 47, 1115–1116. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Reaction of Selenium Tetrabromide with Acetylene. Russ. J. Gen. Chem. 2011, 81, 1241–1242. [Google Scholar] [CrossRef]
- Denmark, S.E.; Collins, W.R.; Cullen, M.D. Observation of Direct Sulfenium and Selenenium Group Transfer from Thiiranium and Seleniranium Ions to Alkenes. J. Am. Chem. Soc. 2009, 131, 3490–3492. [Google Scholar] [CrossRef]
- Borodkin, G.; Chernyak, E.I.; Shakirov, M.M.; Shubin, V.G. On the possibility of nonclassical interaction between episulfonium and episelenonium cycles and the double bond: 1,2,3,3,4,5,6,6-octamethyl-1,4-cyclohexadiene complexes with ArE+ (E = S, Se) electrophilic agents. Russ. J. Org. Chem. 1997, 33, 418–419. [Google Scholar]
- Borodkin, G.; Chernyak, E.I.; Shakirov, M.M.; Shubin, V.G. Possibility of nonclassic reaction between episulfonium and episelenonium cycles and a double bond: P-complexes of 1,2,3,3,4,5,6,6-octamethyl-1,4-cyclohexadiene with cations of RE+ type (E = S, Se). Russ. J. Org. Chem. 1998, 34, 1563–1568. [Google Scholar]
- Wirth, T.; Fragale, G.; Spichty, M. Mechanistic Course of the Asymmetric Methoxyselenenylation Reaction. J. Am. Chem. Soc. 1998, 120, 3376–3381. [Google Scholar] [CrossRef]
- Santi, C.; Fragale, G.; Wirth, T. Synthesis of a new chiral nitrogen containing diselenide as a precursor for selenium electrophiles. Tetrahedron Asymmetry 1998, 9, 3625–3628. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. New Chiral Auxiliaries for Highly Stereoselective Asymmetric Methoxyselenenylations. Org. Lett. 2000, 2, 3007–3009. [Google Scholar] [CrossRef] [PubMed]
- Uehlin, L.; Fragale, G.; Wirth, T. New and Efficient Chiral Selenium Electrophiles. Chem. Eur. J. 2002, 8, 1125–1133. [Google Scholar] [CrossRef]
- Denmark, S.E.; Edwards, M.G. On the Mechanism of the Selenolactonization Reaction with Selenenyl Halides. J. Org. Chem. 2006, 71, 7293–7306. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Nakayama, J. Product class 24: Seleniranes and derivatives. Sci. Synth. 2007, 39, 1023–1032. [Google Scholar]
- Santi, C.; Santoro, S.; Tomassini, C.; Pascolini, F.; Testaferri, L.; Tiecco, M. Enantioselective methoxyselenenylation of α,β-unsaturated aldehydes. Synlett 2009, 5, 743–746. [Google Scholar] [CrossRef]
- Denmark, S.E.; Kalyani, D.; Collins, W.R. Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions. J. Am. Chem. Soc. 2010, 132, 15752–15765. [Google Scholar] [CrossRef] [Green Version]
- Santi, C.; Santoro, S.; Battistelli, B. Organoselenium compounds as catalysts in nature and laboratory. Curr. Org. Chem. 2010, 14, 2442–2462. [Google Scholar] [CrossRef]
- Sancineto, L.; Mangiavacchi, F.; Tidei, C.; Bagnoli, L.; Marini, F.; Gioiello, A.; Scianowski, J.; Santi, C. Selenium-Catalyzed Oxacyclization of Alkenoic Acids and Alkenols. Asian J. Org. Chem. 2017, 6, 988–992. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Santi, C.; Genovese, S. New insights into the seleniranium ion promoted cyclization of prenyl and propenylbenzene aryl ethers. Tetrahedron Lett. 2017, 58, 371–374. [Google Scholar] [CrossRef]
- Poleschner, H.; Seppelt, K. Selenirenium and Tellurirenium Ions. Angew. Chem. Int. Ed. 2008, 47, 6461–6464. [Google Scholar] [CrossRef] [PubMed]
- Poleschner, H.; Seppelt, K. XeF2/Fluoride Acceptors as Versatile One-Electron Oxidants. Angew. Chem. Int. Ed. 2013, 52, 12838–12842. [Google Scholar] [CrossRef] [PubMed]
- Poleschner, H.; Seppelt, K. Seleniranium and Telluriranium Salts. Chem. Eur. J. 2018, 24, 17155–17161. [Google Scholar] [CrossRef]
- Bock, J.; Daniliuc, C.G.; Bergander, K.; Mueck-Lichtenfeld, C.; Hennecke, U. Synthesis, structural characterization, and synthetic application of stable seleniranium ions. Org. Biomol. Chem. 2019, 17, 3181–3185. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |














© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, V.A.; Musalov, M.V.; Kurkutov, E.O.; Yakimov, V.A.; Khabibulina, A.G.; Musalova, M.V.; Amosova, S.V.; Borodina, T.N.; Albanov, A.I. Remarkable Alkene-to-Alkene and Alkene-to-Alkyne Transfer Reactions of Selenium Dibromide and PhSeBr. Stereoselective Addition of Selenium Dihalides to Cycloalkenes. Molecules 2020, 25, 194. https://doi.org/10.3390/molecules25010194
Potapov VA, Musalov MV, Kurkutov EO, Yakimov VA, Khabibulina AG, Musalova MV, Amosova SV, Borodina TN, Albanov AI. Remarkable Alkene-to-Alkene and Alkene-to-Alkyne Transfer Reactions of Selenium Dibromide and PhSeBr. Stereoselective Addition of Selenium Dihalides to Cycloalkenes. Molecules. 2020; 25(1):194. https://doi.org/10.3390/molecules25010194
Chicago/Turabian StylePotapov, Vladimir A., Maxim V. Musalov, Evgeny O. Kurkutov, Vladimir A. Yakimov, Alfiya G. Khabibulina, Maria V. Musalova, Svetlana V. Amosova, Tatyana N. Borodina, and Alexander I. Albanov. 2020. "Remarkable Alkene-to-Alkene and Alkene-to-Alkyne Transfer Reactions of Selenium Dibromide and PhSeBr. Stereoselective Addition of Selenium Dihalides to Cycloalkenes" Molecules 25, no. 1: 194. https://doi.org/10.3390/molecules25010194
APA StylePotapov, V. A., Musalov, M. V., Kurkutov, E. O., Yakimov, V. A., Khabibulina, A. G., Musalova, M. V., Amosova, S. V., Borodina, T. N., & Albanov, A. I. (2020). Remarkable Alkene-to-Alkene and Alkene-to-Alkyne Transfer Reactions of Selenium Dibromide and PhSeBr. Stereoselective Addition of Selenium Dihalides to Cycloalkenes. Molecules, 25(1), 194. https://doi.org/10.3390/molecules25010194