Inhibitory Effect of Osthole from Cnidium monnieri on Tobacco Mosaic Virus (TMV) Infection in Nicotiana glutinosa
Abstract
:1. Introduction
2. Results
2.1. Compound Structure of Osthole
2.2. Anti-TMV Activities of Osthole
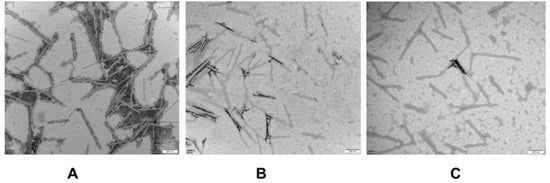
2.3. The Effect of Osthole on Viral Particles
2.4. Kinetic Analysis of the Effect of Osthole Against TMV Infection
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Virus Purification
4.3. Isolation and Purification of Active Compounds and Structure Analysis
4.4. Inhibitory Effect of Osthole on TMV Infection
4.5. Protective Effect of Osthole on TMV Infection
4.6. Curative Effect of Osthole on TMV Infection
4.7. Viral Particle Observation by Transmission Electron Microscope (TEM)
4.8. Western Blot Analysis to Detect the CP of TMV
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, J.; Li, D.B.; Zhou, X.P. The Classification of Plant Viruses, 1st ed.; Beijing Science Press: Beijing, China, 2001; p. 189. [Google Scholar]
- Wu, Y.F. Principles and Methods of Plant Virology, 1st ed.; Xi’an Cartographic Publishing House: Xi’an, China, 1999; pp. 26–30. [Google Scholar]
- Liu, L.R. The Control of Tobacco Diseases and Pests, 1st ed.; Beijing Science Press: Beijing, China, 1998; pp. 10–12. [Google Scholar]
- Liu, Y.; Li, F.; Zhang, S.; Gao, X.; Xie, Y.; Zhang, A.; Dai, L.; Cheng, Z.; Ding, M.; Niu, Y.; et al. Identifcation, distribution and occurrence of viruses in the main vegetables of China. Sci. Agric. Sin. 2019, 52, 239–261. [Google Scholar]
- Wu, Y.F.; Cao, R.; Wei, N.S.; Zhou, G.H. Screening and application of biological virus pesticides. World Pestic. 1995, 5, 35–36. [Google Scholar]
- Fan, H.; Song, B.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Yang, S. Antiviral Activity and Mechanism of Action of Novel Thiourea Containing Chiral Phosphonate on Tobacco Mosaic Virus. Int. J. Mol. Sci. 2011, 12, 4522–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.M.; Wang, L.H.; Li, S.L.; Chen, X.Y.; Shen, Y.M.; Zhang, Z.K.; He, H.P.; Xu, W.B.; Shu, Y.L.; Liang, G.D.; et al. Seco-pregnane steroids target the subgenomic RNA of alphavirus-like RNA viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 8083–8088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Cha, B.; Kim, J.C. Recent trends in studies on botanical fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Feng, C.; Wu, K.; Chen, W.B.; Chen, Y.J.; Hao, X.A.; Wu, Y.F. Advances and prospects in biogenic substances against plant virus: A review. Pestic. Biochem. Phys. 2016, 135, 15–26. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ru, B.L.; Zhai, Y.Y.; Li, J.; Cheng, J.L.; Zhang, Q.; An, D.R. Screening of antiviral activity of extracts from medicinal plants against Tobacco mosaic virus (TMV). Acta Phytophy. Sin. 2018, 45, 463–469. [Google Scholar]
- Grange, M.; Ahmed, S. Handbook of Plants with Pest-Control Properties, 1st ed.; Miss University Press: New York, NY, USA, 1988; p. 470. [Google Scholar]
- Zhu, S.F.; Qiu, W.F. A primary study of the therapeutic effects of some medicinal herb extracts on the pepper mosaic caused by CMV. Acta Phytopathol. Sin. 1989, 19, 123–128. [Google Scholar]
- Jing, B.N.; Ma, Z.Q.; Feng, J.T.; Liang, H.Y.; Li, C.; Zhang, X. Evaluation of the antiviral activity of extracts from plants grown in the Qinling region of China against infection by tobacco mosaic virus (TMV). J. Phytopathol. 2012, 160, 181–186. [Google Scholar] [CrossRef]
- Esam, K.F.E.; Ehab, M.R.M.; Omar, A.A. Antiviral activity of Thuja orientalis extracts against watermelon mosaic virus (WMV) on Citrullus lanatus. Saudi J. Biol. Sci. 2015, 22, 211–219. [Google Scholar]
- Zhao, L.H.; Dong, J.H.; Hu, Z.H.; Li, S.L.; Su, X.X.; Zhang, J.; Yin, Y.Y.; Xu, T.; Zhang, Z.K.; Chen, H.R. Anti-TMV activity and functional mechanisms of two sesquiterpenoids isolated from, Tithonia diversifolia. Pestic. Biochem. Phys. 2017, 140, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, G.H.; Li, Y.H.; Shen, L.L.; Qian, Y.M.; Yang, J.G.; Wang, F.L. Inhibitory effects of sulfated lentinan with different degree of sulfation against tobacco mosaic virus (TMV) in tobacco seedlings. Pestic. Biochem. Phys. 2015, 122, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Yasuda, I.; Kumagai, N.; Tohda, C.; Nojima, H.; Kuraishi, Y.; Komatsu, K. Inhibition of itch-scratch response by fruits of Cnidium monnieri in mice. Biol. Pharm. Bull. 2001, 24, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, J.J.; Xie, M.L.; Zhu, L.J. Treatment of osthol on osteoporosis in ovariectomized rats. Chin. Pharm. Bull. 2011, 27, 591–592. [Google Scholar]
- Matsuda, H.; Ido, Y.; Hirata, A.; Ino, Y.; Naruto, S.; Amamiya, T.; Kubo, M. Antipruritic effect of Cnidii monnieri Fructus (fruits of Cnidium monnieri Cusson). Biol. Pharm. Bull. 2002, 25, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, H.; Tomohiro, N.; Ido, Y.; Kubo, M. Anti-allergic effects of Cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002, 25, 809–812. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.M.; Jia, M.; Li, H.Q.; Zhang, N.D.; Wen, X.; Rahman, K.; Zhang, Q.Y.; Qin, L.P. Cnidium monnieri: A Review of Traditional Uses, Phytochemical and Ethnopharmacological Properties. Am. Chin. Med. 2015, 43, 835–877. [Google Scholar] [CrossRef]
- Kitajima, J.; Aoki, Y.; Ishikawa, T.; Tanaka, Y. Monoterpenoid glucosides of Cnidium monnieri fruit. Chem. Pharm. Bull. 1999, 47, 639–642. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.; Kim, J.S.; Song, E.K.; Cho, H.; Kim, D.H.; Park, S.E.; Lee, H.S.; Kim, Y.C. Sesquiterpenes with hepatoprotective activity from Cnidium monnieri on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med. 2002, 68, 748–749. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Zhou, M.; Liu, Y.; Zhang, G.L.; Luo, Y.G. Chromones and coumarins from the dried fructus of Cnidium monnieri. Fitoterapia 2011, 82, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Gao, C.K.; Li, W.M. Analysis of coumarins in cnidium extracts by UPLC/ESI-TOF-MS/MS. Chin. Pat. Med. 2009, 31, 584–587. [Google Scholar]
- Chappell, J. The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol. 1995, 107, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.Q.; Shen, S.G.; Xu, L.L.; Fan, Y.J. Preliminary study on the inhibitory mechanism of osthol on plant pathogenic fungi. Chin. J. Pestic. Sci. 2004, 4, 28–32. [Google Scholar]
- Wang, C.M.; Su, H.; Chen, H.; Shi, Z.Q.; Fan, Y.J. Mode of action of natural compound eugenol on Tobacco mosaic virus disease. Agrochemicals 2012, 1, 32–34, 39. [Google Scholar]
- Jin, Y.; Hou, L.Y.; Zhang, M.Z.; Tian, Z.F.; Cao, A.C.; Xie, X.M. Antiviral activity of Eupatorium adenophorum leaf extract against tobacco mosaic virus. Crop Prot. 2014, 60, 28–33. [Google Scholar] [CrossRef]
- Hilf, M.E.; Dawson, W.O. The tobamovirus capsid protein functions as a host-specific determinant of long-distance movement. Virology 1993, 193, 106–114. [Google Scholar] [CrossRef]
- Asurmendi, S.; Berg, R.H.; Koo, J.C.; Beachy, R.N. Coat protein regulates formation of replication complexes during tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 1415–1420. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, S.A.; Sarmiento, C.; Valkonen, S.; Truve, E.; Lehto, K. Suppression of infectious TMV genomes expressed in young transgenic tobacco plants. Mol. Plant-Microbe Interact. 1489, 20, 1489–1494. [Google Scholar] [CrossRef]
- Gooding, G.V.; Hebert, T.A. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1289. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |






| Drug | Inhibitory Effect (%) | Protective Effect (%) | Curative Effect (%) |
|---|---|---|---|
| Osthole | 72.57 ± 9.24 aA | 70.26 ± 10.49 aA | 61.97 ± 7.84 aA |
| Eugenol | 60.39 ± 5.48 aA | 56.04 ± 4.98 aA | 60.83 ± 4.49 bB |
| 8% Ningnanmycin SL (1000-X dilution) | 64.11 ± 2.43 aA | 60.57 ± 7.24 aA | 55.45 ± 10.96 aA |
| Concentration (mg/mL) | Inhibitory Effect (%) |
|---|---|
| 1 | 34.46 ± 5.19 cC |
| 3 | 53.23 ± 3.13 bB |
| 5 | 72.57 ± 9.24 aA |
| 7 | 83.22 ± 3.68 aA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Guo, D.-S.; Lu, M.-H.; Yue, J.-Y.; Liu, Y.; Shang, C.-M.; An, D.-R.; Zhao, M.-M. Inhibitory Effect of Osthole from Cnidium monnieri on Tobacco Mosaic Virus (TMV) Infection in Nicotiana glutinosa. Molecules 2020, 25, 65. https://doi.org/10.3390/molecules25010065
Chen Y-H, Guo D-S, Lu M-H, Yue J-Y, Liu Y, Shang C-M, An D-R, Zhao M-M. Inhibitory Effect of Osthole from Cnidium monnieri on Tobacco Mosaic Virus (TMV) Infection in Nicotiana glutinosa. Molecules. 2020; 25(1):65. https://doi.org/10.3390/molecules25010065
Chicago/Turabian StyleChen, Ya-Han, Dong-Sheng Guo, Mei-Huan Lu, Jian-Ying Yue, Yan Liu, Chun-Ming Shang, De-Rong An, and Ming-Min Zhao. 2020. "Inhibitory Effect of Osthole from Cnidium monnieri on Tobacco Mosaic Virus (TMV) Infection in Nicotiana glutinosa" Molecules 25, no. 1: 65. https://doi.org/10.3390/molecules25010065
APA StyleChen, Y.-H., Guo, D.-S., Lu, M.-H., Yue, J.-Y., Liu, Y., Shang, C.-M., An, D.-R., & Zhao, M.-M. (2020). Inhibitory Effect of Osthole from Cnidium monnieri on Tobacco Mosaic Virus (TMV) Infection in Nicotiana glutinosa. Molecules, 25(1), 65. https://doi.org/10.3390/molecules25010065