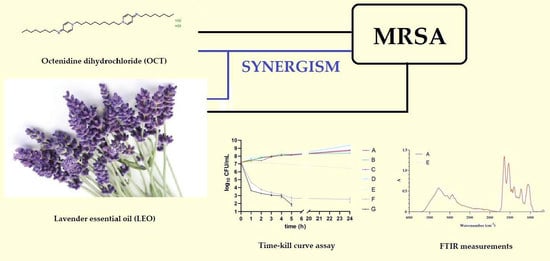
The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of LEO
2.2. The Antibacterial Activity of Chemicals against MRSA Strains
2.3. Synergistic Effect of LEO and OCT
2.4. Effect of LEO Alone and In Combination With OCT against MRSA Reference Strain
2.4.1. Time-Killing Curves
2.4.2. FTIR Analysis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Condition
4.2. Chemicals
4.2.1. Chemical Characterization of LEO
4.2.2. Octenidine Dihydrochloride (OCT)
4.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Chemicals
4.4. Checkerboard Method
4.5. The Influence of LEO Alone and In Combination With OCT on the Chemical Composition of the S. aureus ATCC 43300 (MRSA) Strain
4.5.1. Culture Media Preparation
4.5.2. Time-Kill Curve Assay
4.5.3. A Determination of Functional Groups in Staphylococcal Cells by the Use of Fourier Transform Infrared (FTIR) Spectroscopy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus virulence. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- McCaig, L.F.; McDonald, L.C.; Mandal, S.; Jernigan, D.B. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 2006, 12, 1715–1723. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, Z.; Yang, Z.; Sun, J.; Ma, L. Characterization of community-associated Staphylococcus aureus from skin and soft-tissue infections: A multicenter study in China. Emerg. Microbes Infect. 2016, 5, 127. [Google Scholar] [CrossRef]
- Poggio, J.L. Perioperative strategies to prevent surgical-site infection. Clin. Colon Rectal Surg. 2013, 26, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Velasco, V.; Buyukcangaz, E.; Sherwood, J.S.; Stepan, R.M.; Koslofsky, R.J.; Logue, C.M. Characterization of Staphylococcus aureus from humans and a comparison with isolates of animal origin, in North Dakota, United States. PLoS ONE 2015, 10, 0140497. [Google Scholar] [CrossRef] [Green Version]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [Green Version]
- Hübner, N.O.; Siebert, J.; Kramer, A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar] [CrossRef]
- Szostak, K.; Czogalla, A.; Przybyło, M.; Langner, M. New lipid formulation of octenidine dihydrochloride. J. Liposome Res. 2018, 28, 106–111. [Google Scholar] [CrossRef]
- Eisenbeiβ, W.; Siemers, F.; Amtsberg, G.; Hinz, P.; Hartmann, B.; Kohlmann, T.; Ekkernkamp, A.; Albrecht, U.; Assadian, O.; Kramer, A. Prospective, double-blinded, randomised controlled trial assessing the effect of an octenidine-based hydrogel on bacterial colonisation and epithelialization of skin graft wounds in burn patients. Int. J. Burns Trauma 2012, 2, 71–79. [Google Scholar]
- Hardy, K.; Sunnucks, K.; Gil, H.; Shabir, S.; Trampari, E.; Hawkey, P.; Webber, M. Increased usage of antiseptics is associated with reduced susceptibility in clinical isolates of S. aureus. mBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- De Rapper, S.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid. Based Complement. Alternat. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef] [Green Version]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef] [Green Version]
- Arzi, A.; Ahamehe, M.; Sarahroodi, S. Effect of hydroalcoholic extract of Lavandula officinalis on nicotine-induced convulsion in mice. Pak. J. Biol. Sci. 2011, 14, 634–640. [Google Scholar] [CrossRef]
- Woelk, H.; Schläfke, S. A multi-center, double-blind, randomised study of the lavender oil preparation silexan in comparison to lorazepam for generalized anxiety disorder. Phytomedicine 2010, 17, 94–99. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Romano, A. Inhibitory effect of Lavandula viridis on Fe2+-induced lipid peroxidation, antioxidant and anti-cholinesterase properties. Food Chem. 2011, 126, 1779–1786. [Google Scholar] [CrossRef]
- Messaoud, C.; Chograni, H.; Boussaid, M. Chemical composition and antioxidant activities of essential oils and methanol extracts of three wild Lavandula L. species. Nat. Prod. Res. 2012, 26, 1976–1984. [Google Scholar] [CrossRef]
- Varona, S.; Rojo, S.R.; Martín, Á.; Cocero, M.J.; Serra, A.T.; Crespo, T.; Duarte, C.M.M. Antimicrobial activity of lavandin essential oil formulations against three pathogenic food-borne bacteria. Ind. Crop. Prod. 2013, 42, 243–250. [Google Scholar] [CrossRef]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1359–1366. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Tallian, K. Essential oil of lavender in anxiety disorders: Ready for prime time? Ment. Health Clin. 2018, 7, 147–155. [Google Scholar] [CrossRef]
- ISO 11024-1:1998. Essential oils—General Guidance on Chromatographic Profiles—Part 1: Preparation of Chromatographic Profiles for presentation in Standards. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11024:-1:ed-1:V1:en (accessed on 25 December 2019).
- ISO 11024-2:1998. Essential oils—General Guidance on Chromatographic Profiles—Part 2: Preparation of Chromatographic Profiles for Presentation in Standards. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11024:-2:ed-1:v1:en (accessed on 25 December 2019).
- Kohler, P.; Sommerstein, R.; Schönrath, F.; Ajdler-Schäffler, E.; Anagnostopoulos, A.; Tschirky, S.; Falk, V.; Kuster, S.P.; Sax, H. Effect of perioperative mupirocin and antiseptic body wash on infection rate and causative pathogens in patients undergoing cardiac surgery. Am. J. Infect. Control. 2015, 43, 33–38. [Google Scholar] [CrossRef]
- Şimşek, M.; Duman, R. Investigation of effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacognosy Res. 2017, 9, 234–237. [Google Scholar] [CrossRef] [Green Version]
- Karpanen, T.J.; Worthington, T.; Hendry, E.R.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008, 62, 1031–1036. [Google Scholar] [CrossRef]
- Alabdullatif, M.; Boujezza, I.; Mekni, M.; Taha, M.; Kumaran, D.; Yi, Q.L.; Landoulsi, A.; Ramirez-Arcos, S. Enhancing blood donor skin disinfection using natural oils. Transfusion 2017, 57, 2920–2927. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil its major components to human skin cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Głowacka, A.; Kowalczyk, E.; Wiktorowska-Owczarek, A.; Jóźwiak-Bębenista, M.; Łysakowska, M. The biological activities of cinnamon, geranium and lavender essential oils. Molecules 2014, 19, 20929–20940. [Google Scholar] [CrossRef]
- Honório, V.G.; Bezerra, J.; Souza, G.T.; Carvalho, R.J.; Gomes-Neto, N.J.; Figueiredo, R.C.; Melo, J.V.; Souza, E.L.; Magnani, M. Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Front. Microbiol. 2015, 6, 1223. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.A.; Menorca, A.; van Zuylen, E.M.; Cheung, C.Y.; McConnell, M.A.; Rennison, D.; Brimble, M.A.; Bodle, K.; McDougall, S.; Cook, G.M.; et al. Microtiter screening reveals oxygen-dependent antimicrobial activity of natural products against mastitis-causing bacteria. Front. Microbiol. 2019, 10, 1995. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of Tween 80 on growth and biofilm formation in laboratory media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef]
- Amiali, N.M.; Golding, G.R.; Sedman, J.; Simor, A.E.; Ismail, A.A. Rapid identification of community-associated methicillin-resistant Staphylococcus aureus by Fourier transform infrared spectroscopy. Diagn. Microbiol. Infect. Dis. 2011, 70, 157–166. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dołęgowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The influence of essential oil compounds on antibacterial activity of mupirocin-susceptible and induced low-level mupirocin-resistant MRSA strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef] [Green Version]
- Assadian, O.; Pilcher, M.; Antunes, J.N.P.; Boulton, Z.; von Hallern, B.; Hämmerle, G.; Hunt, G.; Jeffery, S.; Lahnsteiner, E.; Price, J.; et al. Facilitating wound bed preparation: Properties and clinical efficacy of octenidine and octenidine-based products in modern wound management. J. Wound Care 2016, 25, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines (Basel) 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; Heo, H.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.J. Antibacterial activity of essential oil from lavender (Lavandula angustifolia) against pet turtle-borne pathogenic bacteria. Lab. Anim. Res. 2017, 33, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Hochmuth, D. Mass Spectral Library “Terpenoids and Related Constituents of Essential oils”; Library of MassFinder 3: Hamburg, Germany, 2006. [Google Scholar]
- Wiley Science Solutions. Wiley Registry of Mass Spectral Data, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Stein, S.E. NIST 1. NIST/EPA/NIH Mass Spectral LibraryNIST ’98 ASCII Version; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Kwiatkowski, P.; Pruss, A.; Grygorcewicz, B.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Kochan, E.; Sienkiewicz, M. Preliminary study on the antibacterial activity of essential oils alone and in combination with gentamicin against extended-spectrum β-lactamase-producing and New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae isolates. Microb. Drug Resist. 2018, 24, 1368–1375. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Wu, X.; Sun, Y.; Liu, Z. Effect of thyme essential oil against Bacillus cereus planktonic growth and biofilm formation. Appl. Microbiol. Biotechnol. 2018, 102, 10209–10218. [Google Scholar] [CrossRef]
- Orsini, F.; Ami, D.; Villa, A.M.; Sala, G.; Bellotti, M.G.; Doglia, S.M. FT-IR microspectroscopy for microbiological studies. J. Microbiol. Methods 2000, 42, 17–27. [Google Scholar] [CrossRef]
- Salachna, P.; Łopusiewicz, Ł.; Meller, E.; Grzeszczuk, M.; Piechocki, R. Modulation of bioelement concentration and macromolecule conformation in leaves of Salvia coccinea by salicylic acid and salt stress. Fresenius Environ. Bull. 2019, 28, 9339–9347. [Google Scholar]
Sample Availability: Samples of the compounds (Lavandula angustifolia essential oil and octenidine dihydrochloride) are available from the authors. |





| Compound | RI | Relative Concentration (%) |
|---|---|---|
| Monoterpenes | ||
| α-Pinene | 936 | 0.1 |
| Camphene | 950 | 0.1 |
| Myrcene | 987 | 2.4 |
| p-Cymene | 1015 | 0.2 |
| 1,8-Cineole | 1024 | 2.5 |
| Limonene | 1025 | 0.6 |
| (Z)-β-Ocimene | 1029 | 3.2 |
| (E)-β-Ocimene | 1041 | 2.7 |
| γ-Terpinene | 1051 | 0.1 |
| Terpinolene | 1082 | 0.2 |
| Monoterpene isoprenoids | ||
| Linalool | 1086 | 34.1 |
| Camphor | 1123 | 1.2 |
| Izoborneol | 1142 | 0.2 |
| Borneol | 1150 | 1.4 |
| Lavandulol | 1151 | 1.1 |
| Terpinene-4-ol | 1164 | 2.5 |
| cis-Dihydrocarvone | 1172 | 0.2 |
| α-Terpineol | 1176 | 1.8 |
| Linalyl acetate | 1239 | 33.3 |
| Lavandulyl acetate | 1275 | 3.2 |
| Neryl acetate | 1342 | 0.8 |
| Geranyl acetate | 1362 | 1.3 |
| Sesquiterpenes | ||
| β-Caryophyllene | 1421 | 2.7 |
| Aromadendrene | 1443 | 0.1 |
| (E)-β-Farnezene | 1446 | 0.4 |
| Bicyclosesquiphellandrene | 1487 | 0.1 |
| Sesquiterpene isoprenoids | ||
| Caryophyllene oxide | 1578 | 0.1 |
| Esters | ||
| Oct-1-en-3-yl acetate | 1093 | 0.6 |
| Ketones | ||
| Octan-3-one | 969 | 1.3 |
| Total | 98.5 | |
| Bacteria | OCT-LEO | MICo | MBC | MICc | FIC | FICI | Type of Interaction | |
|---|---|---|---|---|---|---|---|---|
| reference strain | ATCC 43300 | OCT (µg/mL) | 1.95 ± 0.00 | 5.21 ± 2.26 | 0.12 ± 0.00 | 0.06 | 0.11 | synergy |
| LEO (mg/mL) | 18.29 ± 7.92 | 439.00 ± 0.00 | 0.86 ± 0.00 | 0.05 | ||||
| isolates | 1 | OCT (µg/mL) | 3.91 ± 0.00 | 11.72 ± 5.52 | 0.12 ± 0.00 | 0.03 | 0.16 | synergy |
| LEO (mg/mL) | 13.72 ± 0.00 | 27.44 ± 0.00 | 1.71 ± 0.00 | 0.13 | ||||
| 2 | OCT (µg/mL) | 3.52 ± 0.00 | 7.04 ± 0.00 | 0.24 ± 0.00 | 0.13 | 0.26 | synergy | |
| LEO (mg/mL) | 13.72 ± 0.00 | 27.44 ± 0.00 | 1.71 ± 0.00 | 0.13 | ||||
| 3 | OCT (µg/mL) | 3.52 ± 0.00 | 7.04 ± 0.00 | 0.12 ± 0.00 | 0.06 | 0.12 | synergy | |
| LEO (mg/mL) | 13.72 ± 0.00 | 27.44 ± 0.00 | 0.86 ± 0.00 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2020, 25, 95. https://doi.org/10.3390/molecules25010095
Kwiatkowski P, Łopusiewicz Ł, Kostek M, Drozłowska E, Pruss A, Wojciuk B, Sienkiewicz M, Zielińska-Bliźniewska H, Dołęgowska B. The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules. 2020; 25(1):95. https://doi.org/10.3390/molecules25010095
Chicago/Turabian StyleKwiatkowski, Paweł, Łukasz Łopusiewicz, Mateusz Kostek, Emilia Drozłowska, Agata Pruss, Bartosz Wojciuk, Monika Sienkiewicz, Hanna Zielińska-Bliźniewska, and Barbara Dołęgowska. 2020. "The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains" Molecules 25, no. 1: 95. https://doi.org/10.3390/molecules25010095
APA StyleKwiatkowski, P., Łopusiewicz, Ł., Kostek, M., Drozłowska, E., Pruss, A., Wojciuk, B., Sienkiewicz, M., Zielińska-Bliźniewska, H., & Dołęgowska, B. (2020). The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules, 25(1), 95. https://doi.org/10.3390/molecules25010095