N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and Their Analogues: Synthesis and Multitarget Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Inhibition of Acetyl- and Butyrylcholinesterase
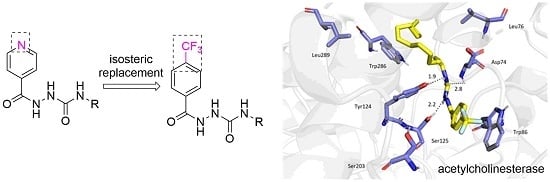
Molecular Docking Study
2.3. Antimycobacterial Activity
2.4. Cytostatic Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of N-alkyl Hydrazine-1-carboxamides 2
Method A
Method B
Method C
3.1.3. Synthesis of 1,2-diacylhydrazines 3
Method A
Method B
3.1.4. Synthesis of 1,3,4-oxadiazole 4
3.2. Product Characterization
3.3. Determination of AChE and BuChE Inhibition
3.4. Molecular Docking
3.5. Antimycobacterial Activity
3.6. Cytostatic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef]
- Hamada, Y.; Kiso, Y. The application of bioisosteres in drug design for novel drug discovery: Focusing on acid protease inhibitors. Expert Opin. Drug Discov. 2012, 7, 903–922. [Google Scholar] [CrossRef]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Bősze, S.; Baranyai, Z.; Stolaříková, J.; Vinšová, J. Synthesis and biological evolution of hydrazones derived from 4-(trifluoromethyl)benzohydrazide. Bioorg. Med. Chem. Lett. 2017, 27, 5185–5189. [Google Scholar] [CrossRef] [PubMed]
- Jamadar, A.; Duhme-Klair, A.K.; Vemuri, K.; Sritharan, M.; Dandawatec, P.; Padhye, S. Synthesis, characterisation and antitubercular activities of a series of pyruvate-containing aroylhydrazones and their Cu-complexes. Dalton Trans. 2012, 41, 9192–9201. [Google Scholar] [CrossRef]
- Vavříková, E.; Polanc, S.; Kočevar, M.; Horváti, K.; Bősze, S.; Stolaříková, J.; Vávrová, K.; Vinšová, J. New fluorine-containing hydrazones active against MDR-tuberculosis. Eur. J. Med. Chem. 2011, 46, 4937–4945. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhong, M.; Zhang, T.; Wu, W.; Wu, Z.; Yang, J.; Xiao, Y.; Pan, Z.; Qiu, G.; Hu, X. Synthesis and anticonvulsant activity of N-3-arylamide substituted 5,5-cyclopropanespirohydantoin derivatives. Eur. J. Med. Chem. 2010, 45, 5870–5877. [Google Scholar] [CrossRef]
- He, X.; Zhong, M.; Zhang, T.; Wu, W.; Wu, Z.; Xiao, Y.; Hu, X. Synthesis and anticonvulsant activity of ethyl 1-(2-arylhydrazinecarboxamido)-2,2-dimethylcyclopropanecarboxylate derivatives. Eur. J. Med. Chem. 2012, 54, 542–548. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.; Soars, S.M.; Kamps, J.; Yin, H. Discovery of Novel Small-Molecule Inhibitors of NF-κB Signaling with Antiinflammatory and Anticancer Properties. J. Med. Chem. 2018, 61, 5881–5899. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Structure–Activity Relationships of Novel Iron Chelators for the Treatment of Iron Overload Disease: The Methyl Pyrazinylketone Isonicotinoyl Hydrazone Series. J. Med. Chem. 2008, 51, 331–344. [Google Scholar] [CrossRef]
- Vosátka, R.; Krátký, M.; Švarcová, M.; Janoušek, J.; Stolaříková, J.; Madacki, J.; Huszár, S.; Mikušová, K.; Korduláková, J.; Trejtnar, F.; et al. New lipophilic isoniazid derivatives and their 1,3,4-oxadiazole analogues: Synthesis, antimycobacterial activity and investigation of their mechanism of action. Eur. J. Med. Chem. 2018, 151, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Rychtarčíková, Z.; Krátký, M.; Gazvoda, M.; Komlóová, M.; Polanc, S.; Kočevar, M.; Stolaříková, J.; Vinšová, J. N-Substituted 2-Isonicotinoylhydrazinecarboxamides — New Antimycobacterial Active Molecules. Molecules 2014, 19, 3851–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krátký, M.; Štěpánková, Š.; Houngbedji, N.-H.; Vosátka, R.; Vorčáková, K.; Vinšová, J. 2-Hydroxy-N-phenylbenzamides and Their Esters Inhibit Acetylcholinesterase and Butyrylcholinesterase. Biomolecules 2019, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Rahim, F.; Ullah, H.; Taha, M.; Wadood, A.; Javed, M.T.; Rehman, W.; Nawaz, M.; Ashraf, M.; Ali, M.; Sajid, M.; et al. Synthesis and in vitro acetylcholinesterase and butyrylcholinesterase inhibitory potential of hydrazide based Schiff bases. Bioorg. Chem. 2016, 68, 30–40. [Google Scholar] [CrossRef]
- Krátký, M.; Štěpánková, Š.; Vorčáková, K.; Švarcová, M.; Vinšová, J. Novel Cholinesterase Inhibitors Based on O-Aromatic N,N-Disubstituted Carbamates and Thiocarbamates. Molecules 2016, 21, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdrazilova, P.; Stepankova, S.; Komers, K.; Ventura, K.; Cegan, A. Half-inhibition concentrations of new cholinesterase inhibitors. Z. Nat. C 2004, 59, 293–296. [Google Scholar]
- Imramovsky, A.; Stepankova, S.; Vanco, J.; Pauk, K.; Monreal-Ferriz, J.; Vinsova, J.; Jampilek, J. Acetylcholinesterase-Inhibiting Activity of Salicylanilide N-Alkylcarbamates and Their Molecular Docking. Molecules 2012, 17, 10142–10158. [Google Scholar] [CrossRef]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2020, 25, 745. [Google Scholar] [CrossRef] [Green Version]
- Sinko, G.; Calic, M.; Bosak, A.; Kovarik, Z. Limitation of the Ellman method: Cholinesterase activity measurement in the presence of oximes. Anal. Biochem. 2007, 370, 223–227. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krátký, M.; Vinšová, J.; Novotná, E.; Mandíková, J.; Trejtnar, F.; Stolaříková, J. Antibacterial Activity of Salicylanilide 4-(Trifluoromethyl)-benzoates. Molecules 2013, 18, 3674–3688. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |








 | ||||
|---|---|---|---|---|
| Code | n | IC50 AChE (µM) | IC50 BuChE (µM) | Selectivity BuChE/AChE |
| 2a | 0 | 31.23 ± 1.87 | 84.16 ± 2.10 | 2.7 |
| 2b | 1 | 56.32 ± 0.54 | 102.80 ± 2.75 | 1.8 |
| 2c | 2 | 82.27 ± 3.31 | 112.70 ± 0.98 | 1.4 |
| 2d | 3 | 38.60 ± 1.32 | 87.81 ± 7.96 | 2.3 |
| 2e | 4 | 43.59 ± 0.41 | 58.01 ± 0.78 | 1.3 |
| 2f | 5 | 49.16 ± 2.36 | 72.47 ± 1.51 | 1.5 |
| 2g | 6 | 59.16 ± 2.38 | 79.29 ± 1.53 | 1.3 |
| 2h | 7 | 76.97 ± 4.75 | 101.28 ± 0.99 | 1.3 |
| 2i | 8 | 106.75 ± 1.73 | 82.24 ± 3.42 | 0.8 |
| 2j | 9 | 49.47 ± 1.74 | 191.81 ± 6.83 | 3.9 |
| 2k | 10 | 40.71 ± 0.37 | 179.12 ± 2.96 | 4.4 |
| 2l | 11 | 45.25 ± 0.69 | 264.14 ± 0.22 | 5.8 |
| 2m | 12 | 28.90 ± 0.67 | 277.48 ± 10.27 | 9.6 |
| 2n | 13 | 38.74 ± 1.14 | 261.70 ± 17.20 | 6.8 |
| 2o | 14 | 27.04 ± 1.13 | 233.18 ± 15.69 | 8.6 |
| 2p | 15 | 68.63 ± 0.56 | 186.75 ± 13.24 | 2.7 |
| 2q | 17 | 72.31 ± 2.01 | 145.72 ± 2.60 | 2.0 |
 | ||||
| 3a | 4 | 67.12 ± 3.05 | 109.98 ± 0.20 | 1.6 |
| 3b | 14 | 91.87 ± 6.79 | 148.60 ± 0.36 | 1.6 |
 | ||||
| 4 | - | 71.32 ± 0.63 | 118.40 ± 1.46 | 1.7 |
| 1 | - | 69.37 ± 1.38 | 204.00 ± 2.69 | 2.9 |
| Rivastigmine | 56.10 ± 1.41 | 38.40 ± 1.97 | 0.7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krátký, M.; Baranyai, Z.; Štěpánková, Š.; Svrčková, K.; Švarcová, M.; Stolaříková, J.; Horváth, L.; Bősze, S.; Vinšová, J. N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and Their Analogues: Synthesis and Multitarget Biological Activity. Molecules 2020, 25, 2268. https://doi.org/10.3390/molecules25102268
Krátký M, Baranyai Z, Štěpánková Š, Svrčková K, Švarcová M, Stolaříková J, Horváth L, Bősze S, Vinšová J. N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and Their Analogues: Synthesis and Multitarget Biological Activity. Molecules. 2020; 25(10):2268. https://doi.org/10.3390/molecules25102268
Chicago/Turabian StyleKrátký, Martin, Zsuzsa Baranyai, Šárka Štěpánková, Katarína Svrčková, Markéta Švarcová, Jiřina Stolaříková, Lilla Horváth, Szilvia Bősze, and Jarmila Vinšová. 2020. "N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and Their Analogues: Synthesis and Multitarget Biological Activity" Molecules 25, no. 10: 2268. https://doi.org/10.3390/molecules25102268
APA StyleKrátký, M., Baranyai, Z., Štěpánková, Š., Svrčková, K., Švarcová, M., Stolaříková, J., Horváth, L., Bősze, S., & Vinšová, J. (2020). N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and Their Analogues: Synthesis and Multitarget Biological Activity. Molecules, 25(10), 2268. https://doi.org/10.3390/molecules25102268