Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials
Abstract
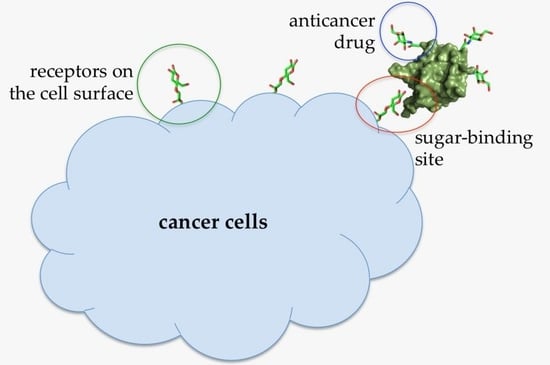
:1. Introduction
2. Lectins and Lectin-Like Protein in A. bisporus
3. Agaricus Bisporus Lectin (ABL)
3.1. Morphology, Characteristics, and Genetics
3.2. Structure and Possible Function in the Mushroom
4. Agaricus bisporus Mannose-Binding Protein (Abmb)
4.1. Morphology, Characteristics, and Genetics
4.2. Structure and Possible Function in the Mushroom
5. Potential Therapeutic Application of Lectin and Lectin-Like Protein from A. bisporus
5.1. ABL
5.2. Abmb
5.3. Potential Therapeutic Application from the Perspective of Other Lectins
6. Future Development of Abmb
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and biological properties of Agaricus bisporus fruiting bodies—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Eswari, S.; Saravana Bhavan, P.; Kalpana, R.; Dharani, C.; Manjula, T.; Sarumathi, K.; Rajkumar, G. Phytochemical characterization of the mushroom Agaricus bisporus and assessment of its nutritional ability in the place of fishmeal for survival and growth of the freshwater prawn Macrobrachium rosenbergii post-larvae. Integr. Food Nutr. Metab. 2019, 6, 1–11. [Google Scholar]
- Dhamodharan, G.; Mirunalini, S. A Novel Medicinal Characterization of Agaricus Bisporus (White Button Mushroom). Pharmacologyonline 2010, 2, 456–463. [Google Scholar]
- Weijn, A.; Bastiaan-Net, S.; Wichers, H.J.; Mes, J.J. Melanin biosynthesis pathway in Agaricus bisporus mushrooms. Fungal Genet. Biol. 2013, 55, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.G.; Fernig, D.G.; White, M.R.; Spiller, D.G.; Appleton, P.; Evans, R.C.; Grierson, I.; Smith, J.A.; Davies, H.; Gerasimenko, O.V.; et al. Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks nuclear localization sequence-dependent nuclear protein import. J. Biol. Chem. 1999, 274, 4890–4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef]
- Hassan, M.A.A.; Rouf, R.; Tiralongo, E.; May, T.W.; Tiralongo, J. Mushroom lectins: Specificity, structure and bioactivity relevant to human disease. Int. J. Mol. Sci. 2015, 16, 7802–7838. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.J.; Dodd, J.E. Hautbergue GM. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.F.S.; Silva MDCd Napoleaão, T.H.; Paiva, P.M.G.; Correia, M.T.S.; Coelho, L.C.B.B. Lectins: Function, structure, biological properties and potential applications. Curr. Top. Pep. Protein Res. 2014, 15, 41–62. [Google Scholar]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef]
- Muthuraman, M.; Koirala, N.; Ciolac, D.; Pintea, B.; Glaser, M.; Groppa, S.; Tamás, G.; Groppa, S. Deep brain stimulation and L-DOPA therapy: Concepts of action and clinical applications in Parkinson’s disease. Front. Neurol. 2018, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Tief, K.; Schmidt, A.; Beermann, F. New evidence for presence of tyrosinase in substantia nigra, forebrain and midbrain. Brain Res. Mol. Brain Res. 1998, 53, 307–310. [Google Scholar] [CrossRef]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978, 14, 633–643. [Google Scholar] [PubMed]
- Ghazarian, H.; Idoni, B.; Oppenheimer, S.B. A glycobiology review: Carbohydrates, lectins, and implications in cancer therapeutics. Acta Histochem. 2011, 113, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorakshakar, A.C.; Ghosh, K. Use of lectins in immunohematology. Asian J. Tranfus. Sci. 2016, 10, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.B.; Drickamer, K. Lectin-like proteins in model organisms: Implications for evolution of carbohydrate-binding activity. Glycobiology 2001, 11, 71R–79R. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Khan, M.I. Fungal lectins: Current molecular and biochemical perspectives. Int. J. Biol. Chem. 2011, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Bansal, R.; Rastogi, A.K.; Kidwai, J.R. Effect of PHA-B fraction of Agaricus bisporus lectin on insulin release and 45Ca2+ uptake by islets of Langerhans in vitro. Acta Diabetol. Lat. 1984, 21, 63–70. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Tjandrawinata, R.R.; Dijkstra, B.W.; Beintema, J.J.; Rachmawati, R.R. Relationship of Agaricus bisporus mannose-binding protein to lectins with β-trefoil fold. Biochem. Biophys. Res. Commun. 2020. In Press. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Yunita, E.A.; Lai, X.; Retnoningrum, D.S.; Rachmawati, H.; Dijkstra, B.W.; Tjandrawinata, R.R. A novel immune-tolerable and permeable lectin-like protein from mushroom Agaricus. Bisporus. Biochem. Biophys Res. Commun. 2016, 473, 1090–1093. [Google Scholar] [CrossRef]
- Wichers, H.J.; Recourt, K.; Hendriks, M.; Ebbelaar, C.E.M.; Biancone, G.; Hoeberichts, F.A.; Mooibroek, H.; Soler-Rivas, C. Cloning, expression and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl. Microbiol. Biotech. 2003, 61, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauracher, S.; Molitor, C.; Michael, C.; Kragl, M.; Rizzi, A.; Rompel, A. High level protein-purification allows the unambiguous polypeptide determination of latent isoform PPO4 of mushroom tyrosinase. Phytochemistry 2014, 99, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komárek, J.; Kavková, E.I.; Houser, J.; Horáčková, A.; Ždánská, J.; Demo, G.; Wimmerová, M. Structure and properties of AB21, a novel Agaricus bisporus protein with structural relation to bacterial pore-forming toxins. Proteins Struct. Func. Bioinf. 2018, 86, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.; Kohler, A.; Baker, A.R.; Foulongne-Oriol, M.; Lombard, V.; Nagye, L.G.; Ohm, R.A. Genome sequence of the button mushroom Agaricus Bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 2012, 109, 17501–17506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crenshaw, R.W.; Harper, S.N.; Moyer, M.; Privalle, L.S. Isolation and Characterization of a cDNA Clone Encoding a Lectin Gene from Agaricus bisporus. Plant Physiol. 1995, 107, 1465–1466. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Van Holle, S.; Van Damme, E.J.M. Messages from the past: New insights in plant lectin evolution. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Barre, A.; Bourne, Y.; Van Damme, E.J.M.; Rougé, P. Overview of the structure–function relationships of mannose-specific lectins from plants, algae and fungi. Int. J. Mol. Sci. 2019, 20, 254. [Google Scholar] [CrossRef] [Green Version]
- Grahn, E.; Askarieh, G.; Holmner, A.; Tateno, H.; Winter, H.C.; Goldstein, I.J.; Krengel, U. Crystal structure of the marasmius oreades mushroom lectin in complex with a xenotransplantation epitope. J. Mol. Biol. 2007, 369, 710–721. [Google Scholar] [CrossRef]
- Ji, S.; Samara, N.L.; Revoredo, L.; Zhang, L.; Tran, D.T.; Muirhead, K.; abak, L.A.; Ten Hagen, K.G. A molecular switch orchestrates enzyme specificity and secretory granule morphology. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Schnaar, R.L. R-Type Lectins. In Essentials of Glycobiology, 3rd ed.; A Varki, R.D.C., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., Schnaar, R.L., Seeberger, P.H., Eds.; Cold Spring Harbor: New York, NY, USA, 2017. [Google Scholar]
- Murzin, A.G.; Lesk, A.M.; Chothia, C. Beta-Trefoil fold. Patterns of structure and sequence in the Kunitz inhibitors interleukins-1 beta and 1 alpha and fibroblast growth factors. J. Mol. Biol. 1992, 223, 531–543. [Google Scholar] [CrossRef]
- Hazes, B. The (QxW)3 domain: A flexible lectin scaffold. Prot. Sci. 1996, 5, 1490–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CAZypedia, T.C. Ten years of CAZypedia: A living encyclopedia of carbohydrate-active enzymes. Glycobiology 2017, 28, 3–8. [Google Scholar]
- Yarbrough, J.M.; Mitta, A.; Mansfield, E.; Taylor, L.E.; Hobdey, S.E.; Sammond, D.W.; Bomble, Y.J.; Crowley, M.F.; Decker, S.R.; Himmel, M.E.; et al. New perspective on glycoside hydrolase binding to lignin from pretreated corn stover. Biotechnol. Biofuels. 2015, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Rachmawati, H.; Sundari, S.; Nabila, N.; Tandrasasmita, O.M.; Amalia, R.; Siahaan, T.J.; Tjandrawinata, R.R.; Ismaya, W.T. Orf239342 from the mushroom Agaricus bisporus is a mannose binding protein. Biochem. Biophys. Res. Commun. 2019, 515, 99–103. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Tandrasasmita, O.M.; Sundari, S.; Lai, X.; Retnoningrum, D.S.; Dijkstra, B.W.; Tjandrawinata, R.R.; Rachmawati, H. The light subunit of mushroom Agaricus bisporus tyrosinase: Its biological characteristics and implications. Int. J. Biol. Macromol. 2017, 102, 308–314. [Google Scholar] [CrossRef]
- Sueyoshi, S.; Tsuji, T.; Osawa, T. Purification and characterization of four isolaectins of mushroom (Agaricus bisporus). Biol. Chem. 1985, 366, 213–221. [Google Scholar]
- Carrizo, M.E.; Irazoqui, F.J.; Lardone, R.D.; Nores, G.A.; Curtino, J.A.; Capaldi, S.; Perduca, M.; Monaco, H.L. Crystallization and preliminary X-ray study of the common edible mushroom (Agaricus bisporus) lectin. Acta Crystallogr. 2004, D60, 718–720. [Google Scholar] [CrossRef]
- Carrizo, M.E.; Capaldi, S.; Perduca, M.; Irazoqui, F.J.; Nores, G.A.; Monaco, H.L. The antineoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J. Biol. Chem. 2005, 280, 10614–10623. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Ma, Z.; Ramachandran, S. Evolutionary history and stress regulation of the lectin superfamily in higher plants. BMC Evol. Biol. 2010, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristan, K.Č.; GabriellaViero Serra, M.D.; Maček, P.; Anderluh, G. Molecular mechanism of pore formation by actinoporins. Toxicon 2009, 54, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System; Delano Scientific LLC: Palo Alto, CA, USA, 2008. [Google Scholar]
- Varrot, A.; Basheer, S.M.; Imberty, A. Fungal lectins: Structure, function and potential applications. Curr. Opin. Struct. Biol. 2013, 2013, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Schurink, M.; Boeriu, C.G.; Wichers, H.; Dijkstra, B.W. Crystallization and preliminary X-ray crystallographic analysis of tyrosinase from the mushroom Agaricus bisporus. Acta Cryst. 2011, F67, 575–578. [Google Scholar]
- Flurkey, A.; Cooksey, J.; Reddy, A.; Spoonmore, K.; Rescigno, A.; Inlow, J.; Flurkey, W.H. Enzyme, protein, carbohydrate, and phenolic contaminants in commercial tyrosinase preparations: Potential problems affecting tyrosinase activity and inhibition studies. J. Agric. Food Chem. 2008, 56, 4760–4768. [Google Scholar] [CrossRef]
- Fujii, Y.; Gerdol, M.; Hasan, I.; Koide, Y.; Matsuzaki, R.; Ikeda, M.; Rajia, S.; Ogawa, Y.; Ozeki, Y. Phylogeny and properties of a novel lectin family with β-trefoil folding in mussels. Trends Glycosci. Glyc. 2018, 30, E195–E208. [Google Scholar] [CrossRef] [Green Version]
- Nabila, N.; Meidianto, V.F.; Tjandrawinata, R.R.; Rachmawati, H.; Ismaya, W.T. Agaricus bisporus mannose binding protein is not an agglutinating protein. Biochem. Biophys. Res. Commun. 2019, 519, 773–776. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Yunita Damayanti, S.; Caroline Tjandrawinata, R.R.; Retnoningrum, D.S.; Rachmawati, H. In silico study to develop a lectin-like protein from mushroom Agaricus bisporus for pharmaceutical application. Sci. Pharm. 2016, 84, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Soler-Lopez, M.; Ismaya, W.T.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of recombinant MtaL at 1.35 Angstrom resolution. Acta Crystallogr. 2016, F72, 244–250. [Google Scholar]
- Miyashita, S.-I.; Sagane, Y.; Suzuki, T.; Matsumoto, T.; Niwa, K.; Watanabe, T. “Non-Toxic” Proteins of the Botulinum Toxin Complex Exert In-vivo Toxicity. Sci. Rep. 2016, 6, 31043. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Casadevall, A. Impact of Melanin on Microbial Virulence and Clinical Resistance to Antimicrobial Compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Fernig, D.G.; Smith, J.A.; Milton, J.D.; Rhodes, J.M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993, 153, 4627–4632. [Google Scholar]
- Parslew, R.; Jones, K.; Rhodes, J.; Sharp, G.R. The antiproliferative effect of lectin from the edible mushroom (Agaricus bisporus) on human keratinocytes: Preliminary studies on its use in psoriasis. Br. J. Dermatol. 1999, 140, 56–60. [Google Scholar] [CrossRef]
- Batterbury, M.; Tebbs, C.A.; Rhodes, J.M.; Grierson, I. Agaricus bisporus (edible mushroom lectin) inhibits ocular fibroblast proliferation and collagen lattice contraction. Exp. Eye Res. 2002, 74, 361–370. [Google Scholar] [CrossRef]
- Cheung, Y.-H.; Sheridan, C.M.; Lo, A.C.Y.; Lai, W.W. Lectin from Agaricus bisporus Inhibited S Phase Cell Population and Akt Phosphorylation in Human RPE Cells. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7469–7475. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.; Wang, H.; Li, C.; Qi, P.; Bao, J. Agaricus bisporus lectins mediates islet β-cell proliferation through regulation of cell cycle proteins. Experiment. Biol. Med. 2012, 237, 287–296. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001, 67, 669–672. [Google Scholar] [CrossRef]
- Greene, W.; Fleisher, T.; Waldmann, T. Suppression of human T and B lymphocyte activation by Agaricus bisporus lectin. I. Suggestive evidence for a surface “suppressor” receptor in human lymphocytes. J. Immunol. 1981, 126, 580–586. [Google Scholar]
- Ditamo, Y.; Rupil, L.L.; Sendra, V.G.; Nores, G.A.; Roth, G.A.; Irazoqui, F.J. In vivo immunomodulatory effect of the lectin from edible mushroom Agaricus bisporus. Food Funct. 2016, 7, 262–269. [Google Scholar] [CrossRef]
- Diana, D.; Ismaya, W.T.; Meidianto, V.F.; Tandrasasmita, O.M.; Tjandrawinata, R.R.; Rachmawati, H. Bioconjugation of captopril-light subunit of Agaricus bisporus mushroom tyrosinase: Characterization and potential use as a drug carrier for oral delivery. Biol. Pharm. Bull. 2018, 41, 1837–1842. [Google Scholar] [CrossRef]
- Haltner, E.; Easson, J.H.; Lehr, C.-M. Lectins and bacterial invasion factors for controlling endo- and transcytosis of bioadhesive drug carrier systems. Eur. J. Pharm. Biopharm. 1997, 44, 3–13. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Efthyani, A.; Retnoningrum, D.S.; Lai, X.; Dijkstra, B.W.; Tjandrawinata, R.R.; Rachmawati, H. Study of response of Swiss Webster mice to light subunit of mushroom tyrosinase. Biotech. Histochem. 2017, 92, 411–416. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Efthyani, A.; Tjandrawinata, R.R.; Rachmawati, H. Biological responses in Balb/c mice after long-term parenteral administration of the light subunit of mushroom tyrosinase. J. Biochem. Mol. Toxicol. 2017, 31, e21958. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. BioMed. Res. Int. 2018, 2018, 3750646–3750657. [Google Scholar] [CrossRef]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with potential for anti-cancer therapy. Molecules. 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [Green Version]
- Hoe, K.K.; Verma, C.S.; Lane, D.P. Drugging the p53 pathway: Understanding the route to clinical efficacy. Nat. Rev. Drug Discov. 2014, 13, 217–236. [Google Scholar]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-targeted drug delivery with mannose-functionalized nanoparticles self-assembled from amphiphilic b-cyclodextrins. Chem. Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef]
- Jamieson, D.; Sunter, N.; Muro, S.; Pouche, L.; Cresti, N.; Lee, J.; ludden, J.; Griffin, M.J.; Allan, J.M.; Verrill, M.W.; et al. Pharmacogenetic association of MBL2 and CD95 polymorphisms with grade 3 infection following adjuvant therapy for breast cancer with doxorubicin and cyclophosphamide. Eur. J. Cancer. 2017, 71, 15–24. [Google Scholar] [CrossRef]
- Kang, H.; Driscoll, H.; Mammoto, A.; Watters, A.L.; Melakeberhan, B.; Diaz, A.; Super, M.; Ingber, D.E. An engineered human Fc-mannose-binding-lectin captures circulating tumor cells. Adv. Biosys. 2017, 1, 1700094–1700100. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirta Ismaya, W.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules 2020, 25, 2368. https://doi.org/10.3390/molecules25102368
Tirta Ismaya W, Tjandrawinata RR, Rachmawati H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules. 2020; 25(10):2368. https://doi.org/10.3390/molecules25102368
Chicago/Turabian StyleTirta Ismaya, Wangsa, Raymond Rubianto Tjandrawinata, and Heni Rachmawati. 2020. "Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials" Molecules 25, no. 10: 2368. https://doi.org/10.3390/molecules25102368
APA StyleTirta Ismaya, W., Tjandrawinata, R. R., & Rachmawati, H. (2020). Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules, 25(10), 2368. https://doi.org/10.3390/molecules25102368