Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics
Abstract
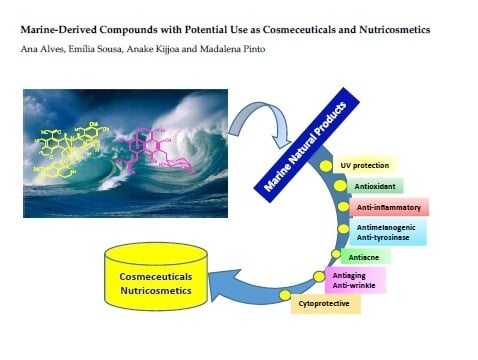
:1. Introduction
2. Biological Targets and Mechanisms of Action of Cosmeceuticals
2.1. Antimelanogenic Activity
Anti-Tyrosinase Activity
2.2. Antiaging Activity
2.2.1. Antiphotoaging Activity
2.2.2. Anti-Wrinkle Activity
2.3. Antioxidant Activity
2.4. Antiacne Activity
2.5. Wound Healing and Anti-Inflammatory Activities
3. Cosmeceuticals from Marine Origin
3.1. Macroalgae-Derived Compounds
3.2. Marine Invertebrate-Derived Compounds
3.2.1. Marine Sponge-Derived Compounds
3.2.2. Coral-Derived Compounds
3.2.3. Sea Cucumber-Derived Compounds
3.3. Marine Microorganisms-Derived Compounds
3.3.1. Microalgae-Derived Compounds
3.3.2. Marine Bacteria-Derived Compounds
3.3.3. Marine Fungi-Derived Compounds
3.3.4. Yeasts-Derived Compounds
4. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guillerme:, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi-an unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Kilgman, A. Cosmeceuticals: A broad-spectrum category between cosmetics and drugs. In Cosmeceuticals and Active Cosmetics, Drug versus Cosmetics; Elsner, P., Maibach, H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 1–9. [Google Scholar]
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioproc. E 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Siahaan, E.; Pangestuti, R.; Munandar, H.; Kim, S.-K. Cosmeceuticals Properties of Sea Cucumbers: Prospects and Trends. Cosmetics 2017, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Marine fungi: An untapped bioresource for future cosmeceuticals. Phytochem. Lett. 2018, 23, 15–20. [Google Scholar] [CrossRef]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef]
- Babitha, S.; Kim, E.-K. Effect of marine cosmeceuticals on the pigmentation of skin. In Marine Cosmeceuticals Trends and Prospects; Kim, S.-K., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; p. 63. [Google Scholar]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.S.; Choi, J.; Lee, M.-S.; Kim, H.-R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jang, E.J.; Bae, S.Y.; Jeon, J.E.; Park, H.J.; Shin, J.; Lee, S.K. Anti-melanogenic activity of gagunin D, a highly oxygenated diterpenoid from the marine sponge Phorbas sp., via modulating tyrosinase expression and degradation. Mar. Drugs 2016, 14, 212. [Google Scholar] [CrossRef] [Green Version]
- Correia, M.S.; Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Festas, T.C.; Tarafder, A.K.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanin transferred to keratinocytes resides in nondegradative endocytic compartments. J. Investig. Dermatol. 2018, 138, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Yun, J.; Lee, C.K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar] [CrossRef] [Green Version]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Xu, Y.; Stokes, A.H.; Freeman, W.M.; Kumer, S.C.; Vogt, B.A.; Vrana, K.E. Tyrosinase mRNA is expressed in human substantia nigra. Brain Res. Mol. Brain Res. 1997, 45, 159–162. [Google Scholar] [CrossRef]
- Abdallah, M. Melasma, novel treatment modalities. J. Pigment. Disord. 2014, 1, 4. [Google Scholar] [CrossRef]
- Chen, J.S.; Wei, C.I.; Marshall, M.R. Inhibition mechanism of kojic acid on polyphenol oxidase. J. Agric. Food Chem. 1991, 39, 1897–1901. [Google Scholar] [CrossRef]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Baek, N.; Nam, T.-G. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res. Pharm. Sci. 2013, 8, 233–242. [Google Scholar]
- Zuo, A.R.; Dong, H.-H.; Yu, Y.-Y.; Shu, Q.-L.; Zheng, L.-X.; Yu, X.-Y.; Cao, S.-W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [Green Version]
- Asthana, S.; Zucca, P.; Vargiu, A.V.; Sanjust, E.; Ruggerone, P.; Rescigno, A. Structure-Activity Relationship Study of Hydroxycoumarins and Mushroom Tyrosinase. J. Agric. Food Chem. 2015, 63, 7236–7244. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. Ann. N. Y. Acad. Sci. 2006, 1067, 323–331. [Google Scholar] [CrossRef]
- Einarsson, S.; Brynjolfsdottir, A.; Krutmann, J. Pharmaceutical and Cosmetic Use of Extracts from Algae Obtainable from Saline Hot Water Sources. WO Patent 2007/129331 A2, 15 November 2007. [Google Scholar]
- Aruoma, O.I. Nutrition and health aspects of free radicals and antioxidants. Food Chem. Toxicol. 1994, 32, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P. Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 1993, 23, 21–48. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. Camb. Philos. Soc. 1999, 74, 311–345. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.P. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B Biol. 1998, 47, 83–94. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Rivera, G.; Llompart, M.; Garcia-Jares, C.; Lores, M. Identification of unwanted photoproducts of cosmetic preservatives in personal care products under ultraviolet-light using solid-phase microextraction and micro-matrix solid-phase dispersion. J. Chromatogr. A 2015, 1390, 1–12. [Google Scholar] [CrossRef]
- Tsatsou, F.; Trakatelli, M.; Patsatsi, A.; Kalokasidis, K.; Sotiriadis, D. Extrinsic aging: UV-mediated skin carcinogenesis. Dermatoendocrinol 2012, 4, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S. The role of cytokines in photoaging. J. Dermatol. Sci. 2000, 23, S30–S36. [Google Scholar] [CrossRef]
- Pallela, R.; Na-Young, Y.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing. Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, B.; Qian, Z.-J.; Kim, M.-M.; Nam, K.W.; Kim, S.-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105. [Google Scholar] [CrossRef]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, R.M.; Yazar, S.; Young, A.R.; Norval, M.; Gruijl, F.R.; Takizawa, Y.; Rhodes, L.E.; Sinclair, C.A.; Neale, R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.; Birch-Machin, M. Mitochondria’s role in skin ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, R.; Kanimozhi, G.; Madahavan, N.R.; Agilan, B.; Ganesan, M.; Prasad, N.R.; Rathinaraj, P. Alpha-pinene attenuates UVA-induced photoaging through inhibition of matrix metalloproteinases expression in mouse skin. Life Sci. 2019, 217, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Shanmugam, M.; Balupillai, A.; Govindhasamy, K.; Gunaseelan, S.; Muthusamy, G.; Robert, B.M.; Nagarajan, R.P. Ultraviolet radiation-induced carcinogenesis: Mechanisms and experimental models. J. Radiat. Cancer Res. 2017, 8, 4–19. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Gao, Q.; Garcia-Pichel, F. Microbial ultraviolet sunscreens. Nat. Rev. Microbiol. 2011, 9, 791–802. [Google Scholar] [CrossRef]
- Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Richa Sinha, R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Groniger, A.; Sinha, R.P.; Klisch, M.; Hader, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef] [Green Version]
- Richa Sinha, R. UV-Mediated stress and its mitigation in cyanobacteria. IJPAES 2011, 1, 155–166. [Google Scholar]
- Pathak, J.; Pandey, A.; Maurya, P.K.; Rajneesh, R.; Sinha, R.P.; Singh, S.P. Cyanobacterial secondary metabolite scytonemin: A potential photoprotective and pharmaceutical compound. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 17, 638. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Bromme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Sárdy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welgus, H.G. Stromelysin: Structure and Function. Agents Actions Suppl. 1991, 35, 61–67. [Google Scholar]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Fisher, G.J.; Choi, H.C.; Bata-Csorgo, Z.; Shao, Y.; Datta, S.; Wang, Z.Q.; Kang, S.; Voorhees, J.J. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J. Investig. Dermatol. 2001, 117, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Tasaki, K.; Shintani, Y.; Saotome, T.; Andoh, A.; Fujiyama, Y.; Hozawa, S.; Bamba, T. Pro-inflammatory cytokine-induced matrix metalloproteinase-1 (MMP-1) secretion in human pancreatic periacinar myofibroblasts. Pancreatology 2003, 3, 414–421. [Google Scholar] [CrossRef]
- Kang, S.; Chung, J.H.; Lee, J.H.; Fisher, G.J.; Wan, Y.S.; Duell, E.A.; Voorhees, J.J. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J. Investig. Dermatol. 2003, 120, 835–841. [Google Scholar] [CrossRef]
- Inomata, S.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; Kohno, Y.; Fukuda, M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Scharffetter, K.; Wlaschek, M.; Hogg, A.; Bolsen, K.; Schothorst, A.; Goerz, G.; Krieg, T.; Plewig, G. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch. Dermatol. Res. 1991, 283, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Südel, K.M.; Venzke, K.; Mielke, H.; Breitenbach, U.; Mundt, C.; Jaspers, S.; Koop, U.; Sauermann, K.; Knussman-Hartig, E.; Moll, I.; et al. Novel aspects of intrinsic and extrinsic aging of human skin: Beneficial effects of soy extract. Photochem. Photobiol. 2005, 81, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.Y.; Agarwal, A.; Uitto, J.; Mauviel, A. An AP-1 binding sequence is essential for regulation of the human alpha2(I) collagen (COL1A2) promoter activity by transforming growth factor-beta. J. Biol. Chem. 1996, 271, 3272–3278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Kim, S.-K. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: The current situation and future prospects. Mar. Drugs 2009, 7, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Sim, G.S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Alparslan, L.; Sekeroglu, N.; Kijjoa, A. The potential of marine resources in cosmetics. CUPMAP 2018, 1, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincon-Fontan, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Preservative and Irritant Capacity of Biosurfactants From Different Sources: A Comparative Study. J. Pharm. Sci. 2019, 108, 2296–2304. [Google Scholar] [CrossRef]
- Hettiarachchy, N.S.; Glenn, K.C.; Gnanasambandam, R.; Johnson, M. Natural antioxidant extract from eenugreek (Trigonella foenumgraecum) for ground beef patties. J. Food Sci. 1996, 61, 516–519. [Google Scholar] [CrossRef]
- Park, P.J.; Jung, W.K.; Nam, K.S.; Shahidi, F.; Kim, S.K. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001, 78, 651–656. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bland, J.S. Oxidants and antioxidants in clinical medicine: Past, present and future potential. J. Nutr. Environ. Med. 1995, 5, 255–280. [Google Scholar] [CrossRef]
- Neumann, C.A.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; Van Etten, R.A. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 2003, 424, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, J.J.; Dreher, F.; Packer, L. Antioxidant defense systems in skin. J. Toxicol. Cutaneous Ocul. Toxicol. 2002, 21, 119–160. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Fisch, K.M.; Wright, A.D.; Konig, G.M. A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 2003, 69, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Miki, W.; Otaki, N.; Yokoyama, A.; Izumida, H.; Shimidzu, N. Okadaxanthin, a novel C-50-carotenoid from a bacterium, Pseudomonas sp Kk10206c associated with marine sponge, Halichondria-Okadai. Experientia 1994, 50, 684–686. [Google Scholar] [CrossRef]
- Miyashita, K. Function of marine carotenoids. Forum Nutr. 2009, 61, 136–146. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sakata, K.; Watanabe, N.; Yagi, A.; Brinen, L.S.; Clardy, J. Chlorophyllonic acid a methyl-ester, a new chlorophyll-a aelated compound isolated as an antioxidant from short-necked clam, ruditapes philippinarum. Tetrahedron Lett. 1992, 33, 2587–2588. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujisawa, A.; Hara, A.; Dunlap, W.C. An unusual vitamin E constituent (α-tocomonoenol) provides enhanced antioxidant protection in marine organisms adapted to cold-water environments. Proc. Natl. Acad. Sci. USA 2001, 98, 13144–13148. [Google Scholar] [CrossRef] [Green Version]
- Al-Amoudi, O.A.; Mutawie, H.H.; Patel, A.V.; Blunden, G. Chemical composition and antioxidant activities of Jeddah corniche algae, Saudi Arabia. Saudi J. Biol. Sci. 2009, 16, 23–29. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 12 March 2020). [CrossRef] [PubMed] [Green Version]
- Rezanka, T.; Temina, M.; Tolstikov, A.G.; Dembitsky, V.M. Natural microbial UV radiation filters--mycosporine-like amino acids. Folia Microbiol. 2004, 49, 339–352. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.J.; Lee, H.W.; Jung, J. Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem. Photobiol. 2003, 78, 109–113. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial fffects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine carotenoids against oxidative stress: Effects on human health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. In-vitro evaluation of marine derived fungi against Cutibacterium acnes. Anaerobe 2018, 49, 5–13. [Google Scholar] [CrossRef]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron. Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 101, 330–333. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ralifo, P.; Crews, P. A new structural theme in the imidazole-containing alkaloids from a calcareous Leucetta sponge. J. Org. Chem. 2004, 69, 9025–9029. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.; Terada, R.; Nozaki, H. The novel anti-Propionibacterium acnes compound, sargafuran, found in the marine brown alga Sargassum macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Bae, H.-J.; Kim, S.-J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar]
- Park, J.H.; Choi, S.H.; Park, S.J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.M.; Ku, S.K.; Song, C.H. Promoting wound healing using low molecular weight fucoidan in a full-thickness dermal excision rat model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [Green Version]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.B.; Smith, D.R. Topical Anti-inflammatories. In Cosmetic Formulation of Skin Care Products, 1st ed.; Draelos, Z.D., Thaman, L.A., Eds.; Taylor & Francis: New York, NY, USA, 2005; pp. 351–353. [Google Scholar]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Christos, C.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef]
- Reilly, D.M.; Parslew, R.; Sharpe, G.R.; Powell, S.; Green, M.R. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm. Venereol. 2000, 80, 171–174. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- McDonough, A.K.; Curtis, J.R.; Saag, K.G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 2008, 20, 131–137. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine diterpenoids as potential anti-inflammatory agents. Mediators Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [Green Version]
- Masre, S.F.; Yip, G.W.; Sirajudeen, K.N.S.; Ghazal, F.C. Wound healing activity of total sulfated glycosaminoglycan (GAG) from Stichopus vastus and Stichopus hermanni integumental tissue in rats. Int. J. Mol. Med. Adv. Sci. 2010, 6, 49–53. Available online: http://medwelljournals.com/abstract/?doi=ijmmas.2010.49.53 (accessed on 10 March 2020). [CrossRef] [Green Version]
- Subramaniam, B.S.; Amuthan, A.; D’Almeida, P.M.; Arunkumar, H.D. Efficacy of gamat extract in wound healing in albino wistar rats. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 142–145. [Google Scholar]
- Mazliadiyana, M.; Nazrun, A.S.; Isa, N.M. Optimum dose of sea cucumber (Stichopus chloronotus) extract for wound healing. Med. Health 2017, 12, 83–89. [Google Scholar] [CrossRef]
- Ming, S. Investigation on component and pharmacology of sea cucumber. Chin. Tradit. Pat. Med. 2001, 10, 21. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZCYA200110021.htm (accessed on 30 April 2020).
- Gupta, S.; Lawrence, W.T. Wound healing: Normal and abnormal mechanisms and closure techniques. In The Physiologic Basis for Surgery, 4th ed.; O.´Leary, J.P., Tabuenca, A., Capote, L.R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; p. 154. [Google Scholar]
- Zohdi, R.M.; Zakaria, Z.A.B.; Yusof, N.; Mustapha, N.M.; Abdullah, M.N.H. Sea cucumber (Stichopus hermanii) based hydrogel to treat burn wounds in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althunibat, O.Y.; Hashim, R.; Bakhtiar, M.T.; Daud, J.M.; Ikeda, M.-A.; Ibrahim, Z. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009, 37, 376–387. [Google Scholar]
- Fredalina, B.D.; Ridzwan, B.H.; Abidin, A.A.Z.; Kaswandi, M.A.; Zaiton, H.; Zali, I.; Kittakoop, P.; Jais, A.M. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen. Pharmacol. 1999, 33, 337–340. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [Green Version]
- Haryanto, H.; Ogai, K.; Suriadi, S.; Nakagami, G.; Oe, M.; Nakatani, T.; Okuwa, M.; Sanada, H.; Sugama, J. A prospective observational study using sea cucumber and honey as topical therapy for diabetic foot ulcers in Indonesia. J. Wellness Health Care 2017, 41, 41–56. [Google Scholar]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef] [Green Version]
- El Barky, A.R.; Ali, E.; Mohamed, T.M. Marine sea cucumber saponins and diabetes. Austin Pancreat Disord. 2017, 1, 1002. Available online: https://austinpublishinggroup.com/pancreatic-disorders/fulltext/pancreas-v1-id1002.php (accessed on 30 April 2020).
- Usoltseva, R.V.; Anastyuk, S.D.; Surits, V.V.; Shevchenko, N.M.; Thinh, P.D.; Zadorozhny, P.A.; Ermakova, S.P. Comparison of structure and in vitro anticancer activity of native and modified fucoidans from Sargassum feldmannii and S. duplicatum. Int. J. Biol. Macromol. 2019, 124, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Anastyuk, S.D.; Kasprik, A.E.; Zvyagintsev, N.V.; Ermakova, S.P. Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polym. 2019, 221, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of Fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosio, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, J.; Li, P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 170–174. [Google Scholar] [CrossRef]
- Savari, R.; Shafiei, M.; Galehdari, H.; Kesmati, M. Expression of VEGF and TGF-β genes in skin wound healing process induced using phenytoin in male rats. Jundishapur J. Health Sci. 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Kijjoa, A.; Sawangwong, P. Drugs and Cosmetics from the Sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Halvorson, H.O. Aquaculture, marine sciences and oceanography: A confluence. connection. Connect. N. Engl. J. High. Educ. Econ. Dev. 1998, 13, 38. [Google Scholar]
- Uppla, L. A review on active ingredients from marine sources used in cosmetics. SOJ Pharm. Pharm. Sci. 2015, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, M.E. Marine chemical ecology: What’s known and what’s next? J. Exp. Mar. Biol. Ecol. 1996, 200, 103–134. [Google Scholar] [CrossRef]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare-A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Sanghvi, A.M.; Lo, Y.M. Present and potential industrial applications of macro- and microalgae. Recent. Pat. Food Nutr. Agric. 2010, 2, 187–194. [Google Scholar] [CrossRef]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Verdy, C.; Branka, J.-E.; Mekideche, N. Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract. Int. J. Cosmet. Sci. 2011, 33, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Blair, C.D.; Faddah, D.A.; Kieckhaefer, J.E.; Olive, M.; Erdos, M.R.; Nabel, E.G.; Collins, F.S. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Investig. 2011, 121, 2833–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.M.; Mendis, E.; Rajapakse, N.; Lee, S.-H.; Kim, S.-K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.-K.; Kim, M.-M.; Kim, S.-K. Inhibitory Effect of Phlorotannins Isolated from Ecklonia cava on Mushroom Tyrosinase Activity and Melanin Formation in Mouse B16F10 Melanoma Cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.-T.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shi, X.; Kim, S.-K. Inhibition of the expression on MMP-2, 9 and morphological changes via human fibrosarcoma cell line by 6,6’-bieckol from marine alga Ecklonia cava. BMB Rep. 2010, 43, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Moon, W.S.; Choi, J.N.; Do, K.H.; Moon, S.H.; Cho, K.K.; Han, C.-J.; Choi, I.S. Effects of seaweed Laminaria japonica extracts on skin moisturizing activity in vivo. J. Cosmet. Sci. 2013, 64, 193–205. [Google Scholar] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Irhimeh, M.; Falk, N. Macroalgal fucoidan extracts: A new opportunity for marine cosmetics. Cosmetics Toiletries 2007, 122, 55–64. Available online: https://www.cosmeticsandtoiletries.com (accessed on 30 April 2020).
- Kwon, K.S. Flavonoid Glycoside Compound Having Tyrosinase Inhibitory Activity, Derived from An Edible Brown Alga, Hiziki fusiformis. Korean Patent KR100739871B, 20 February 2006. [Google Scholar]
- Couteau, C.; Coiffard, L. Seaweed Application in Cosmetics. In Seaweed in Health and Disease Prevention, 1st ed.; Fleurence, J., Levine, I., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 423–441. [Google Scholar]
- Stutz, C.S.; Schmid, D.; Zülli, F. Use of An Extract from Snow Algae in Cosmetic or Dermatological Formulations. U.S. Patent 8,206,721B2, 26 June 2012. [Google Scholar]
- Kennedy, J.; Baker, P.; Piper, C.; Cotter, P.D.; Walsh, M.; Mooij, M.J.; Bourke, M.B.; Rea, M.C.; O’Connor, P.M.; Ross, R.P.; et al. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from Irish waters. Mar. Biotechnol. 2009, 11, 384–396. [Google Scholar] [CrossRef]
- Lu, X.; Cao, X.; Liu, X.; Jiao, B. Marine microbes-derived anti-bacterial agents. Mini Rev. Med. Chem. 2010, 10, 1077–1090. [Google Scholar] [CrossRef]
- Rahman, H.; Austin, B.; Mitchell, W.J.; Morris, P.C.; Jamieson, D.J.; Adams, D.R.; Spragg, A.M.; Schweizer, M. Novel anti-infective compounds from marine bacteria. Mar. Drugs 2010, 8, 498–518. [Google Scholar] [CrossRef]
- Selvin, J.; Shanmughapriya, S.; Gandhimathi, R.; Seghal Kiran, G.; Rajeetha Ravji, T.; Natarajaseenivasan, K.; Hema, T.A. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl. Microbiol. Biotechnol. 2009, 83, 435–445. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.C.; Rutzler, K. Sponges: An essential component of Caribbean coral reefs. Bull. Mar. Sci. 2001, 69, 535–546. [Google Scholar]
- Lee, Y.K.; Lee, J.-H.; Lee, H.K. Microbial symbiosis in marine sponges. J. Microbiol. 2001, 39, 254–264. [Google Scholar]
- Thoms, C.; Horn, M.; Wagner, M.; Hentschel, U.; Proksch, P. Monitoring microbial diversity and natural product profiles of the sponge Aplysina cavernicola following transplantation. Mar. Biol. 2003, 142, 685–692. [Google Scholar] [CrossRef]
- Townsend, E.; Moni, R.; Quinn, R.; Parsons, P.G. Reversible depigmentation of human melanoma cells by halistanol trisulphate, a novel marine sterol. Melanoma Res. 1992, 1, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Liaaen-Jensen, S.; Renstrøm, B.; Ramdahl, T.; Hallenstvet, M.; Bergquist, P. Carotenoids of marine sponges. Biochem. Syst. Ecol. 1982, 10, 167–174. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Higa, T.; Tanaka, J.-I.; Kitamura, A.; Koyama, T.; Takahashi, M.; Uchida, T. Bioactive compounds from marine sponges. Pure Appl. Chem. 1994, 66, 2227–2230. [Google Scholar] [CrossRef]
- Maoka, T.; Nishino, A.; Yasui, H.; Yamano, Y.; Wada, A. Anti-oxidative activity of mytiloxanthin, a metabolite of fucoxanthin in shellfish and tunicates. Mar. Drugs 2016, 14, 93. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.M.; Mendis, E.; Rajapakse, N.; Lee, S.-H.; Kim, S.-K. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 540–546. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Mahato, A.; Thakur, N.L.; Joardar, S.N.; Mandal, B.B. In vitro and in vivo evaluation of the marine sponge skeleton as a bone mimicking biomaterial. Integr. Biol. 2015, 7, 250–262. [Google Scholar] [CrossRef]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, I.; Parma, L.; Bonasoro, F.; Bavestrello, G.; Cerrano, C.; Carnevali, M.D.C. Mechanical adaptability of a sponge extracellular matrix: Evidence for cellular control of mesohyl stiffness in Chondrosia reniformis Nardo. J. Exp. Biol. 2006, 209, 4436–4443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassini, D.; Parma, L.; Lembo, F.; Candia Carnevali, M.D.; Wilkie, I.C.; Bonasoro, F. The reaction of the sponge Chondrosia reniformis to mechanical stimulation is mediated by the outer epithelium and the release of stiffening factor(s). Zoology 2014, 117, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yanti, C.; Vendy, V.; Hwang, J.-K.K. In vitro antiacne activity of marine sponge Acanthella cavernosa extracts. Int. J. Biol. Pharm. Res. (IJBPR) 2015, 6, 388–392. [Google Scholar]
- Look, S.A.; Fenical, W.; Jacobs, R.S.; Clardy, J. The pseudopterosins: Anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc. Natl. Acad. Sci. USA 1986, 83, 6238–6240. [Google Scholar] [CrossRef] [Green Version]
- Dayan, N.; Grove, G.; Sivalenka, R. Anti-inflammatory activity of pseudopterosins by laser doppler blood flow evaluation. Int. J. Cosmet. Sci. 2009, 31, 480. [Google Scholar] [CrossRef]
- Mayer, A.M.; Jacobson, P.B.; Fenical, W.; Jacobs, R.S.; Glaser, K.B. Pharmacological characterization of the pseudopterosins: Novel anti-inflammatory natural products isolated from the Caribbean soft coral, Pseudopterogorgia elisabethae. Life Sci. 1998, 62, PL401–PL407. [Google Scholar] [CrossRef]
- Correa, H.; Valenzuela, A.L.; Ospina, L.F.; Duque, C. Anti-inflammatory effects of the gorgonian Pseudopterogorgia elisabethae collected at the Islands of Providencia and San Andrés (SW Caribbean). J. Inflamm. 2009, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Ata, A.; Win, H.Y.; Holt, D.; Holloway, P.; Segstro, E.P.; Jayatilake, G.S. New antibacterial diterpenes from Pseudopterogorgia elisabethae. Helv. Chim. Acta 2004, 87, 1090–1098. [Google Scholar] [CrossRef]
- Onumah, N. A novel anti-inflammatory in treatment of acne vulgaris: The pseudopterosins. J. Drugs Dermatol. 2013, 12, 1177–1179. [Google Scholar]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.K.; Hirschhorn, R.; et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; Mclntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Moya, C.E.; Jacobs, R.S. Pseudopterosin A inhibits phagocytosis and alters intracellular calcium turnover in a pertussis toxin sensitive site in Tetrahymena thermophila. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 143, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2017, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, M.J.; Koh, H.B.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Effect of Korean Red Sea cucumber (Stichopus japonicus) on melanogenic protein expression in murine B16 melanoma. Int. J. Pharmacol. 2010, 6, 37–42. [Google Scholar] [CrossRef]
- Lee, M.-O.; Oh, H.-G.; Park, S.-H.; Lee, H.-A.; Sul, J.-D.; Song, J.; Kim, O. Skin Whitening Effects of Sanguisorba officinalis and Stichopus japonicus. Lab. Anim. Res. 2010, 26, 127–132. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.Y.; Hong, S.M.; Kwon, E.H.; Lee, T.K. Skin whitening and anti-corrugation activities of glycoprotein fractions from liquid extracts of boiled sea cucumber. Asian Pac. J. Trop. Med. 2016, 9, 1002–1006. [Google Scholar] [CrossRef]
- Mourão, P.A.; Bastos, I.G. Highly acidic glycans from sea cucumbers. Isolation and fractionation of fucose-rich sulfated polysaccharides from the body wall of Ludwigothurea grisea. Eur. J. Biochem. 1987, 166, 639–645. [Google Scholar] [CrossRef]
- Myron, P.; Siddiquee, S.; Azad, S.A. Fucosylated chondroitin sulfate diversity in sea cucumbers: A review. Carbohydr. Polym. 2014, 112, 173–178. [Google Scholar] [CrossRef]
- Pomin, V.H. Holothurian fucosylated chondroitin sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta 2013, 1830, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Structure-function relationship of anticoagulant and antithrombotic well-defined sulfated polysaccharides from marine invertebrates. Adv. Food Nutr. Res. 2012, 65, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xue, C.; Chang, Y.; Xu, X.; Ge, L.; Liu, G.; Wang, Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014, 146, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, W.M.; Rocher, A. Role of secondary metabolites and pigments in the epidermal tissues, ripe ovaries, viscera, gut contents and diet of the sea cucumber Holothuria atra. Mar. Biol. 1999, 133, 163–169. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M.M.; Kijjoa, A. Anticancer and cancer preventive compounds from edible marine organisms. Semin. Cancer Biol. 2017, 46, 55–64. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C.; Chalker, B.; Banaszak, A.T.; Rosenzweig, T.K. Survey of ultraviolet radiation-absorbing mycosporine-like amino acids in organs of coral reef holothuroids. Mar. Ecol. Prog. Ser. 1992, 90, 139–148. Available online: https://www.int-res.com/articles/meps/90/m090p139.pdf (accessed on 26 April 2020). [CrossRef]
- McClintock, J.; Karentz, D. Mycosporine-like amino acids in 38 species of subtidal marine organisms from McMurdo Sound, Antarctica. Antarct. Sci. 1997, 9, 392–398. [Google Scholar] [CrossRef]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar] [CrossRef]
- Afkhami, M.; Ehsanpour, M. Evaluation bioactivity of a Sea cucumber, Stichopus hermanni from Persian Gulf. J. Exp. Biol. 2014, 4, 254–258. [Google Scholar]
- Ibrahim, H.A.H. Antibacterial carotenoids of three Holothuria species in Hurghada, Egypt. Egypt. J. Aquat. Res. 2012, 38, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, A.J.; Afifi, R.; Ahmed, M.; Khalifa, S.; Paget, T. Bioactivity as an options value of sea cucumbers in the Egyptian Red Sea. Conserv. Biol. 2010, 24, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Masre, S.F.; Yip, G.W.; Sirajudeen, K.N.; Ghazali, F.C. Quantitative analysis of sulphated glycosaminoglycans content of Malaysian sea cucumber Stichopus hermanni and Stichopus vastus. Nat. Prod. Res. 2012, 26, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [Green Version]
- Ireland, C.M.; Copp, B.R.; Mark, P.M.P.; McDonald, L.A.; Radisky, D.C.; Swersey, J.C. Biomedical Potential of Marine Natural Products. In Marine Biotechnology: Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Springer Science +Business Media, LLC: New York, NY, USA, 1993; Volume 1, pp. 1–30. [Google Scholar]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Lanora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef]
- Sousa, I.; Gouveia, L.; Batista, A.P.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food product. In Food Chemistry Research Developments; Konstantinos, N., Papadopoulos, P.P., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2008; pp. 75–112. [Google Scholar]
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial metabolites as a source of sunscreens and moisturizers: A comparison with current synthetic compounds. Eur. J. Phycol. 2017, 52, 43–56. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef]
- Raposo, M.F.D.; Morais, R.M.S.C.; Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2020, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, U.; Sarada, R.; Rao, S.R.; Ravishankar, G.A. Production of astaxanthin in Haematococcus pluvialis cultured in various media. Bioresour. Technol. 1999, 68, 197–199. [Google Scholar] [CrossRef]
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012, 30, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.; Franklin, S. Cosmetic Compositions Comprising Microalgal Components. U.S. Patent 8,557,249 B2, 15 October 2013. [Google Scholar]
- Mourelle, M.L.; Gómez, C.; Soto, J.L.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-T.; Chen, P.-Y.; Wu, J.-J.; Lee, T.-M.; Hsu, S.-L.; Chang, C.-M.J.; Young, C.-C.; Shieh, C.-J. Purification of algal anti-tyrosinase zeaxanthin from Nannochloropsis oculata using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–962. [Google Scholar] [CrossRef]
- Pieber, S.; Schober, S.; Mittelbach, M. Pressurized fluid extraction of polyunsaturated fatty acids from the microalga Nannochloropsis oculata. Biomass Bioenerg. 2012, 47, 474–482. [Google Scholar] [CrossRef]
- Amori, P.; Lotti, J.; Lotti, T.; Vitiello, G. Clinical evaluation of a new cosmetic cream containing PEPHA®-TIGHT on the skin of childbearing women. J. Biol. Regul. Homeost. Agents. 2017, 31, 141–145. [Google Scholar] [PubMed]
- Neumann, U.; Derwenskus, F.; Flister, V.F.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Pudney, A.; Gandini, C.; Economou, C.K.; Smith, R.; Goddard, P.; Napier, J.A.; Spicer, A.; Sayanova, O. Multifunctionalizing the marine diatom Phaeodactylum tricornutum for sustainable co-production of omega-3 long chain polyunsaturated fatty acids and recombinant phytase. Sci. Rep. 2019, 9, 11444. [Google Scholar] [CrossRef] [PubMed]
- Nizard, C.; Friguet, B.; Moreau, M.; Bulteau, A.L.; Saunois, A. Use of Phaeodactylum Algae Extract as Cosmetic Agent Promoting the Proteasome Activity of Skin Cells and Cosmetic Composition Comprising same. U.S. Patent 204/0136945A1, 15 July 2004. [Google Scholar]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, L.; Probert, I.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting marine plankton. Mar Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latasa, M. Pigment composition of Heterocapsa sp. and Thalassiosira weissflogii growing in batch cultures under different irradiances. Sci. Mar. 1995, 59, 25–37. [Google Scholar]
- Ishika, T.; Laird, D.W.; Bahri, P.A.; Moheimani, N.R. Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J. Appl. Phycol. 2019, 31, 1535–1544. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F.; Liu, X.; Li, X.-Z. Carotenoid composition in the green microalga Chlorococcum. Food Chem. 2002, 76, 319–325. [Google Scholar] [CrossRef]
- Prartono, T.; Kawaroe, M.; Katili, V. Fatty acid composition of three diatom species Skeletonema costatum, Thalassiosira sp. and Chaetoceros gracilis. Int. J. Environ. Bioenerg. 2013, 6, 28–43. [Google Scholar]
- Peltomaa, E.; Hällfors, H.; Taipale, S.J. Comparison of diatoms and dinoflagellates from different habitats as sources of PUFAs. Mar. Drugs 2019, 17, 233. [Google Scholar] [CrossRef] [Green Version]
- Zanella, L.; Pertile, P.; Massironi, M.; Massironi, M.; Caviola, E. Extracts of Microalgae and Their Application. U.S. Patent 2019/0117712 A1, 25 April 2019. [Google Scholar]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef]
- Balkrishna, A.; Agarwal, V.; Kumar, G.; Gupta, A.K. Applications of bacterial polysaccharides with special reference to the cosmetic industry. In Microbial Bioprospecting for Sustainable Development; Singh, J., Sharma, D., Kumar, G., Sharma, N.J., Eds.; Springer: Singapore, 2018; pp. 189–202. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [Green Version]
- Costaouec, T.; Cerantola, S.; Ropartz, D.; Ratiskol, J.; Sinquin, C.; Colliec-Jouault, S.; Boisset, C. Structural data on a bacterial exopolysaccharide produced by a deep-sea Alteromonas macleodii strain. Carbohydr. Polym. 2012, 90, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtois, A.; Berthou, C.; Guezennec, J.; Boisset, C.; Bordron, A. Exopolysaccharides isolated from hydrothermal vent bacteria can modulate the complement system. PLoS ONE 2014, 9, e94965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare carotenoids, (3R)-saproxanthin and (3R,2’S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.S.; Choi, T.-J.; Lee, W.J.; Kim, Y.T. Paracoccus haeundaensis sp. nov., a Gram-negative, halophilic, astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1699–1702. [Google Scholar] [CrossRef]
- Yokoyama, A.; Miki, W. Composition and presumed biosynthetic pathway of carotenoids in the astaxanthin-producing bacterium Agrobacterium aurantiacum. FEMS Microbiol. Lett. 1995, 128, 139–144. [Google Scholar] [CrossRef]
- Kang, H.Y.; Yoon, T.J.; Lee, G.J. Whitening effects of marine pseudomonas extract. Ann. Dermatol. 2011, 23, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Deering, R.W.; Chen, J.; Sun, J.; Ma, H.; Dubert, J.; Barja, J.L.; Seeram, N.P.; Wang, H.; Rowley, D.C. N-Acyl dehydrotyrosines, tyrosinase inhibitors from the marine bacterium Thalassotalea sp. PP2-459. J. Nat. Prod. 2016, 79, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Bownik, A.; Stepniewska, Z. Ectoine as a promising protective agent in humans and animals. Arh. Hig. Rada Toksikol. 2016, 67, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Kunte, H.J.; Lentzen, G.; Galinski, E. Industrial production of the cell protectant ectoine: Protection mechanisms, processes, and products. Curr. Biotechnol. 2014, 3, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Marini, A.; Reinelt, K.; Krutmann, J.; Bilstein, A. Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: A randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin Pharmacol. Physiol. 2014, 27, 57–65. [Google Scholar] [CrossRef]
- Heinrich, U.; Garbe, B.; Tronnier, H. In vivo assessment of ectoin: A randomized, vehicle-controlled clinical trial. Skin Pharmacol. Physiol. 2007, 20, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zaccai, G.; Bagyan, I.; Combet, J.; Cuello, G.J.; Deme, B.; Fichou, Y.; Gallat, F.X.; Josa, V.M.G.; Gronau, S.; Haertlein, M.; et al. Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane. Sci. Rep. 2016, 6, 31434. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Anzali, S.; Buenger, J.; Pfluecker, F.; Driller, H. The multifunctional role of ectoine as a natural cell protectant. Clin. Dermatol. 2008, 26, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association--a review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmaraj, S.; Ashokkumar, B.; Dhevendaran, K. Food-grade pigments from Streptomyces sp. isolated from the marine sponge Callyspongia diffusa. Food Res. Int. 2009, 42, 487–492. [Google Scholar] [CrossRef]
- Dharmaraj, S.; Ashokkumar, B.; Dhevendaran, K. Fermentative production of carotenoids from marine actinomycetes. Iran. J. Microbiol. 2009, 1, 36–41. [Google Scholar]
- Kogej, T.; Gostincar, C.; Volkmann, M.; Gorbushina, A.A.; Gunde-Cimerman, N. Mycosporines in extremophilic fungi-Novel complementary osmolytes? Environ. Chem. 2006, 3, 105–110. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.; Kang, J.S.; Choi, H.D.; Son, B.W. Circumdatin I, a new ultraviolet-A protecting benzodiazepine alkaloid from a marine isolate of the fungus Exophiala. J. Antibiot. 2008, 61, 40–42. [Google Scholar] [CrossRef]
- Li, X.; Kim, M.K.; Lee, U.; Kim, S.K.; Kang, J.S.; Choi, H.D.; Son, B.W. Myrothenones A and B, cyclopentenone derivatives with tyrosinase inhibitory activity from the marine-derived fungus Myrothecium sp. Chem. Pharm. Bull. 2005, 53, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.H.; Li, X.G.; Kang, J.S.; Choi, H.D.; Son, B.W. A new α-pyrone derivative, 6-[(E)-hept-1-enyl]-α-pyrone, with tyrosinase inhibitory activity from a marine isolate of the fungus Botrytis. Bull. Korean Chem. Soc. 2007, 28, 887–888. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, T.; Yamada, K.; Minoura, K.; Miyamoto, K.; Usami, Y.; Kobayashi, T.; Hamada-Sato, N.; Imada, C.; Tsujibo, H. Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol. Pharm. Bull. 2008, 31, 1618–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233 Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Jeong, J.H.; Lee, K.T.; Rho, J.R.; Choi, H.D.; Kang, J.S.; Son, B.W. γ-Pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Alternaria sp. Arch. Pharm. Res. 2003, 26, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Kaya, K.; Nakazawa, A.; Matsuura, H.; Honda, D.; Inouye, I.; Watanabe, M.M. Thraustochytrid Aurantiochytrium sp 18W-13a accummulates high amounts of squalene. Biosci. Biotechnol. Biochem. 2011, 75, 2246–2248. [Google Scholar] [CrossRef] [Green Version]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71, 361S–366S. [Google Scholar] [CrossRef] [Green Version]
- Burja, A.M.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, G. Mechanisms of fatty acid synthesis in marine fungus-like protists. Appl. Microbiol. Biotechnol. 2015, 99, 8363–8375. [Google Scholar] [CrossRef]
- Gupta, A.; Barrow, C.J.; Puri, M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 2012, 30, 1733–1745. [Google Scholar] [CrossRef]
- Aasen, I.M.; Ertesvag, H.; Heggeset, T.M.; Liu, B.; Brautaset, T.; Vadstein, O.; Ellingsen, T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016, 100, 4309–4321. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, B.; Liu, D.; Gao, S.; Proksch, P.; Lin, W. Brasilianoids A-F, new meroterpenoids from the sponge-associated fungus Penicillium brasilianum. Front. Chem. 2018, 6, 314. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Tschachler, E. Cuts by caspase-14 control the proteolysis of filaggrin. J. Investig. Dermatol. 2011, 131, 2173–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell. Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suen, Y.L.; Tang, H.; Huang, J.; Chen, F. Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. J. Agric. Food Chem. 2014, 62, 12392–12398. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Yao, M.; Gu, X.; Li, B.; Liu, H.; Ding, M.; Xiao, W.; Yuan, Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels 2018, 11, 230. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. https://doi.org/10.3390/molecules25112536
Alves A, Sousa E, Kijjoa A, Pinto M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules. 2020; 25(11):2536. https://doi.org/10.3390/molecules25112536
Chicago/Turabian StyleAlves, Ana, Emília Sousa, Anake Kijjoa, and Madalena Pinto. 2020. "Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics" Molecules 25, no. 11: 2536. https://doi.org/10.3390/molecules25112536
APA StyleAlves, A., Sousa, E., Kijjoa, A., & Pinto, M. (2020). Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules, 25(11), 2536. https://doi.org/10.3390/molecules25112536