Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development
Abstract
:1. Introduction
2. In Vitro Cytotoxicity of C17 and C18 Acetylenic Oxylipins and Structure Activity-Relationship
2.1. Cytotoxic C17 and C18 Acetylenic Oxylipins Isolated from Plants of the Araliaceae
2.1.1. Cytotoxic C17 and C18 Acetylenic Oxylipins from Panax Species
2.1.2. Cytotoxic C17 and C18 Acetylenic Oxylipins from Other Plant Species of the Araliaceae
2.2. Cytotoxic C17 and C18 Acetylenic Oxylipins Isolated from Plants of the Asteraceae
2.3. Cytotoxic C17 and C18 Acetylenic Oxylipins Isolated from Plants of the Apiaceae
2.3.1. Cytotoxic C17 and C18 Acetylenic Oxylipins from Apicaceae Food Plants
2.3.2. Cytotoxic C17 and C18 Acetylenic Oxylipins from Apiaceae Medicinal Plants
2.4. Cytotoxic C17 and C18 Acetylenic Oxylipins from Other Plant Families
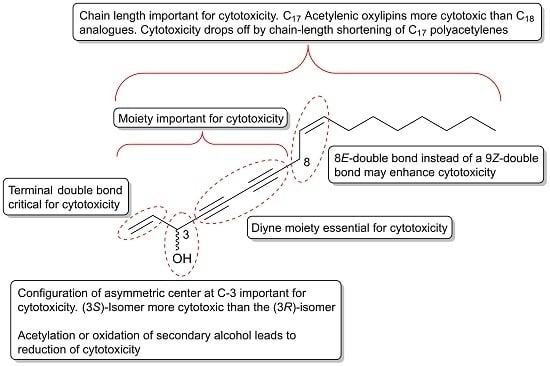
2.5. Moieties and Stereochemistry that Are Important for the Cytotoxicity of C17 and C18 Acetylenic Oxylipins
3. In Vitro Anti-Inflammatory Activity of C17 and C18 Acetylenic Oxylipins
4. In Vivo Anticancer Activity of C17 and C18 Acetylenic Oxylipins
4.1. In Vivo Studies of the Chemopreventive Effect of Falcarinol and Falcarindiol in a Rat Model for CRC
4.2. Studies of the Anticancer Effect of C17 and C18 Acetylenic Oxylipins in Other In Vivo Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bohlmann, F.; Burkhardt, T.; Zdero, C. Naturally Occurring Acetylenes; Academic Press: London, UK, 1973. [Google Scholar]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, L.P.; Jakobsen, H.B. Polyacetylenes: Distribution in Higher Plants, Pharmacological Effects and Analysis. In Thin Layer Chromatography in Phytochemistry. Chromatographic Science Series; Waksmundzka-Hajnos, M., Sherma, J., Kowalska, T., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2008; Volume 99, pp. 757–816. [Google Scholar] [CrossRef]
- Nathalie, L.; Fahmi, E.M.; Mohamed, M.; Philippe, A. Marine polyacetylenes: Distribution, biological properties, and synthesis. Stud. Nat. Prod. Chem. 2015, 45, 251–295. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Anticancer activity of natural and synthetic acetylenic lipids. Lipids 2006, 41, 883–924. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, C.Z.; Yuan, C.S.; Huang, W.H. Oplopanax horridus: Phytochemistry and pharmacological diversity and structure-activity relationship on anticancer effects. Evid. Based Complement Altern. Med. 2018, 2018, 9186926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, L.P. Aliphatic C17-polyacetylenes of the falcarinol-type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anticancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Hansen, L.; Boll, P.M. Polyacetylenes in Araliaceae: Their chemistry, biosynthesis and biological significance. Phytochemistry 1986, 25, 285–293. [Google Scholar] [CrossRef]
- Christensen, L.P. Acetylenes and related compounds in Anthemideae. Phytochemistry 1992, 31, 7–49. [Google Scholar] [CrossRef]
- Christensen, L.P.; Lam, J. Acetylenes and related compounds in Astereae. Phytochemistry 1991, 30, 2453–2476. [Google Scholar] [CrossRef]
- Christensen, L.P.; Lam, J. Acetylenes and related compounds in Heliantheae. Phytochemistry 1991, 30, 11–49. [Google Scholar] [CrossRef]
- Heydenreuter, W.; Kunold, E.; Sieber, S.A. Alkynol natural products target ALDH2 in cancer cells by irreversible binding to the active site. Chem. Commun. 2015, 51, 15784–15787. [Google Scholar] [CrossRef] [Green Version]
- Czyzewska, M.M.; Chrobok, L.; Kania, A.; Jatczak, M.; Pollastro, F.; Appendino, G.; Mozrzymas, J.W. Dietary acetylenic oxylipin falcarinol differentially modulates GABAA receptors. J. Nat. Prod. 2014, 77, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Nakayama, S.; Anan, E.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Activation of the Nrf2/ARE pathway via S-alkylation of cysteine 151 in the chemopreventive agent-sensor Keap1 protein by falcarindiol, a conjugated diacetylene compound. Toxicol. Appl. Pharm. 2010, 244, 27–36. [Google Scholar] [CrossRef]
- Tan, K.W.; Killeen, D.P.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Dietary polyacetylenes of the falcarinol type are inhibitors of breast cancer resistance protein (BCRP/ABCG2). Eur. J. Pharm. 2014, 723, 346–352. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L.; Raduner, S.; Cottiglia, F.; Floris, C.; Altmann, K.-H.; Gertsch, J. Falcarinol is a covalent cannabinoid CB1 receptor antagonist and induces pro-allergic effects in skin. Biochem. Pharmacol. 2010, 79, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Sun, A.; Xie, Y.; Isse, T.; Kawamoto, T.; Zou, Y.; Ge, J. Aldehyde dehydrogenase-2 deficiency aggravates cardiac dysfunction elicited by endoplasmic reticulum stress induction. Mol. Med. 2012, 18, 785–793. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. Febs J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, K.; Yamasaki, D.; Ishimoto, K.; Doi, T. The role of PPARs in cancer. Ppar Res. 2008, 2008, 102737. [Google Scholar] [CrossRef] [Green Version]
- Michalik, L.; Desvergne, B.; Wahli, W. Peroxisome-proliferator-activated receptors and cancers: Complex stories. Nat. Rev. Cancer 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Christensen, L.P.; El-Houri, R.B. Development of an in vitro screening platform for the identification of partial PPARγ agonists as a source for antidiabetic lead compounds. Molecules 2018, 23, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Houri, R.B.; Kotowska, D.; Christensen, K.B.; Bhattacharya, S.; Oksbjerg, N.; Wolber, G.; Kristiansen, K.; Christensen, L.P. Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct. 2015, 6, 2135–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Blunder, M.; Fakhrudin, N.; Liu, X.; Noha, S.M.; Malainer, C.; Kramer, M.P.; Cocic, A.; Kunert, O.; Schinkovitz, A.; et al. Polyacetylenes from Notopterygium incisum—New selective partial agonists of peroxisome proliferator-activated receptor-gamma. PLoS ONE 2013, 8, e61755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanson, A.L.; Bakovic, M. Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxid. Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef]
- Ohnuma, T.; Komatsu, T.; Nakayama, S.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Induction of antioxidant and phase 2 drug-metabolizing enzymes by falcarindiol isolated from Notopterygium incisum extract, which activates the Nrf2/ARE pathway, leads to cytoprotection against oxidative and electrophilic stress. Arch. Biochem. Biophys. 2009, 488, 34–41. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharm. 2013, 85, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, M.L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; El-Rayes, B.F. Cyclooxygenase-2 in gastrointestinal malignancies. Cancer 2019, 125, 1221–1227. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharm. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef]
- Agrawal, U.; Kumari, N.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Overexpression of COX-2 indicates poor survival in urothelial bladder cancer. Ann. Diagn. Pathol. 2018, 34, 50–55. [Google Scholar] [CrossRef]
- Harris, R.E.; Casto, B.C.; Harris, Z.M. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J. Clin. Oncol. 2014, 5, 677–692. [Google Scholar] [CrossRef] [Green Version]
- Petkovaa, D.K.; Clelland, C.; Ronan, J.; Pang, L.; Coulson, J.M.; Lewis, S.; Knox, A.J. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 2004, 98, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Yip-Schneider, M.T.; Barnard, D.S.; Billings, S.D.; Cheng, L.; Heilman, D.K.; Lin, A.; Marshall, S.J.; Crowell, P.L.; Marshall, M.S.; Sweeney, C.J. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 2000, 21, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Srivastava, M.; Ahmad, N.; Bostwick, D.G.; Mukhtar, H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 2000, 42, 73–78. [Google Scholar] [CrossRef]
- Saba, N.F.; Choi, M.; Muller, S.; Shin, H.J.C.; Tighiouart, M.; Papadimitrakopoulou, V.A.; El-Naggar, A.K.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Role of cyclooxygenase-2 in tumor progression and survival of head and neck squamous cell carcinoma. Cancer Prev. Res. 2009, 2, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrec, H.; Ponce, R.; Preston, B.D.; Iles, J.; Born, T.L.; Hooper, M. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr. Med. Res. Opin. 2015, 31, 557–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Shain, K.H.; Yarde, D.N.; Meads, M.B.; Huang, M.; Jove, R.; Hazlehurst, L.A.; Dalton, W.S. β1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: Implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009, 69, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.H.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Khuri, F.R.; Wu, H.; Lee, J.J.; Kemp, B.L.; Lotan, R.; Lippman, S.M.; Feng, L.; Hong, W.K.; Xu, X.C. Cyclooxygenase-2 over-expression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 861–867. [Google Scholar]
- Kobaek-Larsen, M.; Baatrup, G.; Notabi, M.K.; El-Houri, R.B.; Pipó-Ollé, E.; Arnspang, E.C.; Christensen, L.P. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef] [Green Version]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; He, Y.S.; Xu, L.H.; Zhang, B.Y.; Qi, L.W.; Yang, J.; Li, P.; Wen, X.D. Pharmacokinetic profiles of falcarindiol and oplopandiol in rats after oral administration of polyynes extract of Oplopanax elatus. Chin. J. Nat. Med. 2016, 14, 714–720. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Acetylenes and psoralens. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2009; Chapter 5; pp. 147–173. [Google Scholar]
- Brandt, K.; Christensen, L.P.; Hansen-Møller, J.; Hansen, S.L.; Haraldsdóttir, J.; Jespersen, L.; Purup, S.; Kharazmi, A.; Barkholdt, V.; Frøkiær, H.; et al. Health promoting compounds in vegetables and fruits: A systematic approach for identifying plant components with impact on human health. Trends Food Sci. Tech. 2004, 15, 384–393. [Google Scholar] [CrossRef]
- Jin, X.; Che, D.; Zhang, Z.; Yan, H.; Jia, Z.; Jia, X. Ginseng consumption and risk of cancer: A meta-analysis. J. Ginseng Res. 2016, 40, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Seo, E.K.; Gyllenhaal, C.; Block, K.I. Panax ginseng: A role in cancer therapy? Integr. Cancer. 2003, 2, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.K. Panax ginseng—A non-organ-specific cancer preventive? Lancet Oncol. 2001, 2, 49–55. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides: Chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar] [CrossRef]
- Shim, S.C.; Koh, H.Y.; Han, B.H. Polyacetylene compounds from Panax ginseng C. A. Meyer. Bull. Korean Chem. Soc. 1983, 4, 183–188. [Google Scholar]
- Fujimoto, Y.; Satoh, M. Acetylenes from the callus of Panax ginseng. Phytochemistry 1987, 26, 2850–2852. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Satoh, M.; Takeuchi, N.; Kirisawa, M. Synthesis and absolute configurations of the cytotoxic polyacetylenes isolated from the callus of Panax ginseng. Chem. Pharm. Bull. (Tokyo) 1990, 38, 1447–1450. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, Y.; Satoh, M. A new cytotoxic chlorine-containing polyacetylene from the callus of Panax ginseng. Chem. Pharm. Bull. (Tokyo) 1988, 36, 4206–4208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, B.Z. Beziehung zwischen Struktur und cytotoxischer Aktivität von Panaxydol-Analogen gegen L1210 Zellen. Arch. Pharm. 1988, 321, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Katano, M.; Yamamoto, H.; Mori, M.; Takata, K. Studies on the panaxytriol of Panax ginseng C. A. Meyer. Isolation, determination and antitumor activity. Chem. Pharm. Bull. (Tokyo) 1989, 37, 1279–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, H.; Katano, M.; Yamamoto, H.; Fujito, H.; Mori, M.; Takata, K. Cytotoxic activity of polyacetylene compounds in Panax ginseng C. A. Meyer. Chem. Pharm. Bull. (Tokyo) 1990, 38, 3480–3482. [Google Scholar] [CrossRef] [Green Version]
- Hirakua, K.; Takagi, H.; Morita, M.; Nakajima, K.; Niitsu, K.; Sasaki, H.; Maruno, M.; Okada, M. Cytotoxic activity of acetylenic compounds from Panax ginseng. Nat. Med. 2000, 54, 342–345. [Google Scholar]
- Yang, M.C.; Seo, D.S.; Choi, S.U.; Park, Y.H.; Lee, K.R. Polyacetylenes from the roots of cultivated-wild ginseng and their cytotoxicity in vitro. Arch. Pharm. Res. 2008, 31, 154–159. [Google Scholar] [CrossRef]
- Matsunaga, H.; Saita, T.; Nagumo, F.; Mori, M.; Katano, M. Relationship between antiproliferative activity of acetylenic alcohol, panaxydol, and its affinity for target cell membrane. Gan Kagaku Ryoho 1994, 21, 2585–2589. [Google Scholar]
- Moon, J.; Yu, S.J.; Kim, H.S.; Sohn, J. Induction of G1 cell cycle arrest and p27Kip1 increase by panaxydol isolated from Panax ginseng. Biochem. Pharm. 2000, 59, 1109–1116. [Google Scholar] [CrossRef]
- Guo, L.; Song, L.; Wang, Z.; Zhao, W.; Mao, W.; Yin, M. Panaxydol inhibits the proliferation and induces the differentiation of human hepatocarcinoma cell line HepG2. Chem. Biol. Interact. 2009, 181, 138–143. [Google Scholar] [CrossRef]
- Lee, J.H.; Leem, D.G.; Chung, K.S.; Kim, K.T.; Choi, S.Y.; Lee, K.T. Panaxydol derived from Panax ginseng inhibits G1 cell cycle progression in non-small cell lung cancer via upregulation of intracellular Ca2+ levels. Biol. Pharm. Bull. 2018, 41, 1701–1707. [Google Scholar] [CrossRef] [Green Version]
- Hai, J.; Lin, Q.; Lu, Y.; Zhang, H.; Yi, J. Induction of apoptosis in rat C6 glioma cells by panaxydol. Cell. Biol. Int. 2007, 31, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yu, S.J.; Oh, H.J.; Lee, J.Y.; Kim, Y.; Sohn, J. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis 2011, 16, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lim, J.M.; Kim, J.Y.; Kim, Y.; Park, S.; Sohn, J. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int. J. Cancer 2016, 138, 1432–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourougaa, K.; Naski, N.; Boularan, C.; Mlynarczyk, C.; Candeias, M.M.; Marullo, S.; Fåhraeus, R. Endoplasmic reticulum stress induces G2 cell-cycle arrest via mRNA translation of the p53 Isoform p53/47. Mol. Cell 2010, 38, 78–88. [Google Scholar] [CrossRef]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol. 2015, 308, C415–C425. [Google Scholar] [CrossRef] [Green Version]
- Abukhdeir, A.M.; Park, B.H. P21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev. Mol. Med. 2008, 10, e19. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, H.; Saita, T.; Nagumo, F.; Mori, M.; Katano, M. A possible mechanism for the cytotoxicity of a polyacetylenic alcohol, panaxytriol: Inhibition of mitochondrial respiration. Cancer Chemother. Pharm. 1995, 35, 291–296. [Google Scholar] [CrossRef]
- Matsunaga, H.; Katano, M.; Saita, T.; Yamamoto, H.; Mori, M. Potentiation of cytotoxicity of mitomycin C bt a polyacetylenic alcohol, panaxytriol. Cancer Chemother. Pharm. 1994, 33, 291–297. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.W.; Kim, S.H.; Wee, J.J.; Kim, Y.S.; Lee, H.J. Inhibitory effect of tumor cell proliferation and induction of G2/M cell cycle arrest by panaxytriol. Planta Med. 2002, 68, 119–122. [Google Scholar] [CrossRef]
- Satoh, Y.; Satoh, M.; Isobe, K.; Mohri, K.; Yoshida, Y.; Fujimoto, Y. Studies on panax acetylenes: Absolute structure of a new panax acetylene, and inhibitory effects of related acetylenes on the growth of L-1210 cells. Chem. Pharm. Bull. (Tokyo) 2007, 55, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, Y.; Wang, H.; Satoh, M.; Takeuchi, N. Polyacetylenes from Panax quinquefolium. Phytochemistry 1994, 35, 1255–1257. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Wang, H.; Kirisawa, M.; Satoh, M.; Takeuchi, N. Acetylenes from Panax quinquefolium. Phytochemistry 1992, 31, 3499–3501. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Satoh, M.; Takeuchi, N.; Kirisawa, M. Cytotoxic acetylenes from Panax quinquefolium. Chem. Pharm. Bull. (Tokyo) 1991, 39, 521–523. [Google Scholar] [CrossRef] [Green Version]
- Setzer, W.N.; Gu, X.; Wells, E.B.; Setzer, M.C.; Moriarity, D.M. Synthesis and cytotoxic activity of a series of diacetylenic compounds related to falcarindiol. Chem. Pharm. Bull. (Tokyo) 2000, 48, 1776–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washida, D.; Kitanaka, S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem. Pharm. Bull. (Tokyo) 2003, 51, 1314–1317. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Yang, R.; Jiang, Y.; Yang, Z.; Yang, J.; Zhao, Q.; Lu, Y. Induction of apoptosis in human promyelocytic leukemia HL60 cells by panaxynol and panaxydol. Molecules 2011, 16, 5561–5573. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Ding, Y.; Kim, J.H.; Yang, S.Y.; Boo, H.J.; Kang, H.K.; Nguyen, M.C.; Kim, Y.H. Polyacetylenes from Panax stipuleanatus and their cytotoxic effects on human cancer cells. Bull. Korean Chem. Soc. 2011, 32, 3513–3516. [Google Scholar] [CrossRef] [Green Version]
- Tuyen, N.Q.; Hoa, L.T.P.; Huong, L.T.D.; Quang, D.N. Heptadeca-8-en-4,6-diyne-3,10-diol—A new cytotoxic polyacetylene from Vietnamese Panax stipuleanatus. Chem. Nat. Compd. 2018, 54, 156–157. [Google Scholar] [CrossRef]
- McCutcheon, A.R.; Ellis, S.M.; Hancock, R.E.W.; Towers, G.H.N. Antibiotic screening of medicinal plants of the British Columbian native peoples. J. Ethnopharmacol. 1992, 37, 213–223. [Google Scholar] [CrossRef]
- Kobaisy, M.; Abramowski, Z.; Lermer, L.; Saxena, G.; Hancock, R.E.; Towers, G.H.; Doxsee, D.; Stokes, R.W. Antimycobacterial polyynes of Devil’s Club (Oplopanax horridus), a North American native medicinal plant. J. Nat. Prod. 1997, 60, 1210–1213. [Google Scholar] [CrossRef]
- Sun, S.; Du, G.J.; Qi, L.W.; Williams, S.; Wang, C.Z.; Yuan, C.S. Hydrophobic constituents and their potential anticancer activities from Devil’s Club (Oplopanax horridus Miq.). J. Ethnopharmacol. 2010, 132, 280–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.H.; Zhang, Q.W.; Yuan, C.S.; Wang, C.Z.; Li, S.P.; Zhou, H.H. Chemical constituents of the plants from the genus Oplopanax. Chem. Biodivers. 2014, 11, 181–196. [Google Scholar] [CrossRef] [PubMed]
- McGill, C.M.; Alba-Rodriguez, E.J.; Li, S.; Benson, C.J.; Ondrasik, R.M.; Fisher, L.N.; Claxton, D.F.; Barth, B.M. Extracts of Devil’s club (Oplopanax horridus) exert therapeutic efficacy in experimental models of acute myeloid leukemia. Phytother. Res. 2014, 28, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Sun, S.; Du, G.J. Effects of Oplopanax horridus on human colorectal cancer cells. Anticancer Res. 2010, 30, 295–302. [Google Scholar]
- Tai, J.; Cheung, S.; Chan, E.; Hasman, D. Inhibition of human ovarian cancer cell lines by Devil’s club Oplopanax horridus. J. Ethnopharmacol. 2010, 127, 478–485. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.L.; Wang, C.Z.; Williams, S.; Yuan, C.S. Improving anticancer activities of Oplopanax horridus root bark extract by removing water-soluble components. Phytother. Res. 2010, 24, 1166–1174. [Google Scholar] [CrossRef] [Green Version]
- Tai, J.; Cheung, S.; Cheah, S.; Chan, E.; Hasman, D. In vitro antiproliferative and antioxidant studies on Devil’s Club Oplopanax horridus. J. Ethnopharmacol. 2006, 108, 228–235. [Google Scholar] [CrossRef]
- Huang, W.H.; Zhang, Q.W.; Wang, C.Z.; Yuan, C.S.; Li, S.P. Isolation and identification of two new polyynes from a North American ethnic medicinal plant—Oplopanax horridus (Smith) Miq. Molecules 2010, 15, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Z.; Zhang, Z.; Huang, W.H.; Du, G.J.; Wen, X.D.; Calway, T.; Yu, C.; Nass, R.; Zhao, J.; Du, W.; et al. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine 2013, 20, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yu, C.; Zhang, C.F.; Wu, X.H.; Wen, X.D.; Anderson, S.; Du, W.; Huang, W.H.; Li, S.P.; Wang, C.Z.; et al. Chemopreventive effects of oplopantriol A, a novel compound isolated from Oplopanax horridus, on colorectal cancer. Nutrients 2014, 6, 2668–2680. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.Z.; Huang, W.H.; Wang, C.Z.; Yuan, C.S.; Li, S.P. Anticancer activities of polyynes from the root bark of Oplopanax horridus and their acetylated derivatives. Molecules 2014, 19, 6142–6162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, J.; Cheung, S.S.; Ou, D.; Warnock, G.L.; Hasman, D. Antiproliferation activity of Devil’s club (Oplopanax horridus) and anticancer agents on human pancreatic cancer multicellular spheroids. Phytomedicine 2014, 21, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.R.; Liao, Y.; Li, X.; Zhang, Z.; Zhao, J.; Wang, C.Z.; Huang, W.H.; Li, S.P.; Yuan, C.S.; Du, W. Anticancer compound oplopantriol A kills cancer cells through inducing ER stress and BH3 proteins Bim and Noxa. Cell Death Dis. 2014, 5, e1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.Z.; Huang, W.H.; Li, S.P.; He, T.C.; Yuan, C.S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef]
- Cheung, S.S.C.; Hasman, D.; Khelifi, D.; Tai, J.; Smith, R.W.; Warnock, G.L. Devil’s Club falcarinol-type polyacetylenes inhibit pancreatic cancer cell proliferation. Nutr. Cancer 2019, 71, 301–311. [Google Scholar] [CrossRef]
- Yang, M.C.; Kwon, H.C.; Kim, Y.J.; Lee, K.R.; Yang, H.O. Oploxynes A and B, polyacetylenes from the stems of Oplopanax elatus. J. Nat. Prod. 2010, 73, 801–805. [Google Scholar] [CrossRef]
- Yadav, J.S.; Boyapelly, K.; Alugubelli, S.R.; Pabbaraja, S.; Vangala, J.R.; Kalivendi, S.V. Stereoselective total synthesis of (+)-oploxyne A, (−)-oploxyne B, and their C-10 epimers and structure revision of natural oploxyne B. J. Org. Chem. 2011, 76, 2568–2576. [Google Scholar] [CrossRef]
- Ohnuma, T.; Anan, E.; Hoashi, R.; Takeda, Y.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Dietary diacetylene falcarindiol induces phase 2 drug-metabolizing enzymes and blocks carbon tetrachloride-induced hepatotoxicity in mice through suppression of lipid peroxidation. Biol. Pharm Bull. 2011, 34, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Bernart, M.W.; Cardellina, J.H.; Balaschak, M.S.; Alexander, M.R.; Shoemaker, R.H.; Boyd, M.R. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 1996, 59, 748–753. [Google Scholar] [CrossRef]
- Yamazoe, S.; Hasegawa, K.; Shigemori, H. Growth inhibitory indole acetic acid polyacetylenic ester from Japanese ivy (Hedera rhombea Bean). Phytochemistry 2007, 68, 1706–1711. [Google Scholar] [CrossRef]
- Tsolmon, S.; Kurita, Y.; Yamada, P.; Shigemori, H.; Isoda, H. Indoleacetic acid falcarindiol ester induces granulocytic differentiation of the human leukemia cell line HL-60. Planta Med. 2009, 75, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lo, J.M.; Chan, Y.F. Cytotoxic components from the leaves of Schefflera taiwaniana. J. Chin. Chem. Soc. 2002, 49, 427–431. [Google Scholar] [CrossRef]
- Jung, H.J.; Min, B.S.; Park, J.Y.; Kim, Y.H.; Lee, H.K.; Bae, K.H. Gymnasterkoreaynes A–F, cytotoxic polyacetylenes from Gymnaster koraiensis. J. Nat. Prod. 2002, 65, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Cai, X.F.; Shen, Q.; Lee, I.S.; Lee, E.J.; Park, Y.K.; Bae, K.; Kim, Y.H. Gymnasterkoreayne G, a new inhibitory polyacetylene against NFAT transcription factor from Gymnaster koraiensis. Chem. Pharm. Bull. (Tokyo) 2005, 53, 1194–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.K.; Kim, K.H.; Ryu, S.Y.; Choi, S.U.; Lee, K.R. Phytochemical constituents from the flowers of Gymnaster koraiensis and their cytotoxic activities in vitro. Bull. Korean Chem. Soc. 2010, 31, 227–229. [Google Scholar] [CrossRef] [Green Version]
- Carpita, A.; Braconi, S.; Rossi, R. The first total synthesis of naturally occurring (+)-gymnasterkoreayne F and its enantiomer. Tetrahedron Asymmetry 2005, 16, 2501–2508. [Google Scholar] [CrossRef]
- Lee, S.B.; Kang, K.; Oidovsambuu, S.; Jho, E.H.; Yun, J.H.; Yoo, J.H.; Lee, E.H.; Pan, C.H.; Lee, J.K.; Jung, S.H.; et al. A polyacetylene from Gymnaster koraiensis exerts hepatoprotective effects in vivo and in vitro. Food Chem. Toxicol. 2010, 48, 3035–3041. [Google Scholar] [CrossRef]
- Lee, K.M.; Kang, K.; Lee, S.B.; Nho, C.W. Nuclear factor-E2 (Nrf2) is regulated through the differential activation of ERK1/2 and PKC α/βII by Gymnasterkoreayne B. Cancer Lett. 2013, 330, 225–232. [Google Scholar] [CrossRef]
- Shin, D.; Yang, J.E.; Lee, S.B.; Nho, C.W. SAR studies of gymnasterkoreayne derivatives with cancer chemopreventive activities. Bioorg. Med. Chem. Lett. 2010, 20, 7549–7552. [Google Scholar] [CrossRef]
- Takaishi, Y.; Okuyama, T.; Nakano, K.; Murakami, K.; Tomimatsu, T. Absolute configuration of a triolacetylene from Cirsium japonicum. Phytochemistry 1991, 30, 2321–2324. [Google Scholar] [CrossRef]
- Takaishi, Y.; Okuyama, T.; Masuda, A.; Nakano, K.; Murakami, K.; Tomimatsu, T. Acetylenes from Cirsium japonicum. Phytochemistry 1990, 29, 3849–3852. [Google Scholar] [CrossRef]
- Stavri, M.; Ford, C.H.; Bucar, F.; Streit, B.; Hall, M.L.; Williamson, R.T.; Mathew, K.T.; Gibbons, S. Bioactive constituents of Artemisia monosperma. Phytochemistry 2005, 66, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cheng, Y.; Li, S.; Zhu, Y.; Xu, X.; Zheng, X.; Mao, Q.; Xie, L. Dietary carrot consumption and the risk of prostate cancer. Eur. J. Nutr. 2014, 53, 1615–1623. [Google Scholar] [CrossRef]
- Fallahzadeh, H.; Jalali, A.; Momayyezi, M.; Bazm, S. Effect of carrot intake in the prevention of gastric cancer: A meta-analysis. J. Gastric Cancer 2015, 15, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Shao, F.; Zhang, F.; Miao, Q. Association between dietary carrot intake and breast cancer: A meta-analysis. Medicine 2018, 97, e12164. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, H.; Yang, W.; Song, F.; Yan, S.; Wang, C.; Fu, W.; Li, H.; Lyu, C.; Gan, Y.; et al. Is carrot consumption associated with a decreased risk of lung cancer? A meta-analysis of observational studies. Br. J. Nutr. 2019, 122, 488–498. [Google Scholar] [CrossRef]
- Deding, U.; Baatrup, G.; Christensen, L.P.; Kobaek-Larsen, M. Carrot intake and risk of colorectal cancer: A prospective cohort study of 57,053 Danes. Nutrients 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C₁₇-polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agri. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Christensen, L.P.; Theil, P.K.; Oksbjerg, N. The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose-Response 2008, 6, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Regul. Toxicol. Pharm. 2011, 61, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharm. Sci. 2001, 22, 285–291. [Google Scholar] [CrossRef]
- Kong, C.S.; Umb, Y.R.; Lee, J.I.; Kim, Y.A.; Yea, S.S.; Seo, Y. Constituents isolated from Glehnia littoralis suppress proliferations of human cancer cells and MMP expression in HT1080 cells. Food Chem. 2010, 120, 385–394. [Google Scholar] [CrossRef]
- Um, Y.R.; Kong, C.S.; Lee, J.I.; Kim, Y.A.; Nam, T.J.; Seo, Y. Evaluation of chemical constituents from Glehnia littoralis for antiproliferative activity against HT-29 human colon cancer cells. Process Biochem. 2010, 45, 114–119. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, Y.L.; Huang, C.P.; Shu, J.W.; Tsai, W.J. A tumor cell growth inhibitor from Saposhnikovae divaricata. Cancer Invest. 2002, 20, 955–964. [Google Scholar] [CrossRef]
- Lu, T.; Gu, M.; Zhao, Y.; Zheng, X.; Xing, C. Autophagy contributes to falcarindiol-induced cell death in breast cancer cells with enhanced endoplasmic reticulum stress. PLoS ONE 2017, 12, e0176348. [Google Scholar] [CrossRef]
- Kim, T.J.; Kwon, H.S.; Kang, M.; Leem, H.H.; Lee, K.H.; Kim, D.Y. The antitumor natural compound falcarindiol disrupts neural stem cell homeostasis by suppressing Notch pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. Aaps J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.H.; Leem, M.J.; Shin, D.H.; Chang, H.B.; Hong, S.W.; Moon, E.Y.; Lee, D.K.; Yoon, S.J.; Woo, W.S. Cytotoxic constituents from the roots of Anthriscus sylvestris. Arch. Pharm. Res. 1999, 22, 208–212. [Google Scholar] [CrossRef]
- Ikeda, R.; Nagao, T.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents in umbelliferae plants. IV. Constituents in the fruits of Anthriscus sylvestris Hoffm. Chem. Pharm. Bull. (Tokyo) 1998, 46, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Lee, G.; Park, H.G.; Choi, M.L.; Kim, Y.H.; Park, Y.B.; Song, K.S.; Cheong, C.; Bae, Y.S. Falcarindiol, a polyacetylenic compound isolated from Peucedanum japonicum, inhibits mammalian DNA topoisomerase I. J. Microbiol. Biotechnol. 2000, 10, 394–398. [Google Scholar]
- Govindan, G.; Sambandan, T.G.; Govindan, M.; Sinskey, A.; Vanessendelft, J.; Adenan, I.; Rha, C.K. A bioactive polyacetylene compound isolated from Centella asiatica. Planta Med. 2007, 73, 597–599. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Zhang, W.; Su, J. Toxic polyacetylenes in the genus Bupleurum (Apiaceae)—Distribution, toxicity, molecular mechanism and analysis. J. Ethnopharmacol. 2016, 193, 566–573. [Google Scholar] [CrossRef]
- Huang, H.Q.; Zhang, X.; Shen, Y.H.; Su, J.; Liu, X.H.; Tian, J.M.; Lin, S.; Shan, L.; Zhang, W.D. Polyacetylenes from Bupleurum longiradiatum. J. Nat. Prod. 2009, 72, 2153–2157. [Google Scholar] [CrossRef]
- Appendino, G.; Pollastro, F.; Verotta, L.; Ballero, M.; Romano, A.; Wyrembek, P.; Szczuraszek, K.; Mozrzymas, J.W.; Taglialatela-Scafati, O. Polyacetylenes from Sardinian Oenanthe fistulosa: A molecular clue to risus sardonicus. J. Nat. Prod. 2009, 72, 962–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommerwerk, S.; Heller, L.; Siewert, B.; Csuk, R. Chemoenzymatic synthesis and cytotoxicity of oenanthotoxin and analogues. Bioorg. Med. Chem. 2015, 23, 5595–5602. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Lee, K.H. Antitumor agents, 85. Circutoxin, an antileukemic principle from Circuta maculate, and the cytotoxicity of the related derivatives. J. Nat. Prod. 1986, 49, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Hadacek, F.; Wurz, G.; Teuscher, E.; Greger, H. Polyacetylenes from water hemlock, Cicuta virosa. Planta Med. 1995, 61, 439–445. [Google Scholar] [CrossRef]
- Liu, W.; Yang, B.; Yang, L.; Kaur, J.; Jessop, C.; Fadhil, R.; Good, D.; Ni, G.; Liu, X.; Mosaiab, T.; et al. Therapeutic effects of ten commonly used Chinese herbs and their bioactive compounds on cancers. Evid. Based Complement. Altern. Med. 2019, 2019, 6057837. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.C.; Lee, J.; Jin, W.; Youn, U.; Kim, H.; Lee, I.S.; Zhang, X.; Song, K.; Seong, Y.; Bae, K. Cytotoxic constituents from angelicae sinensis radix. Arch. Pharm. Res. 2007, 30, 565–569. [Google Scholar] [CrossRef]
- Furumi, K.; Fujioka, T.; Fujii, H.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M.; Mihashi, K. Novel antiproliferative falcarindiol furanocoumarin ethers from the root of Angelica japonica. Bioorg. Med. Chem. Lett. 1998, 8, 93–96. [Google Scholar] [CrossRef]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents from umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem. Pharm. Bull. (Tokyo) 1999, 47, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Dall’Acqua, S.; Viola, G.; Piacente, S.; Cappelletti, E.M.; Innocenti, G. Cytotoxic constituents of roots of Chaerophyllum hirsutum. J. Nat. Prod. 2004, 67, 1588–1590. [Google Scholar] [CrossRef]
- Bae, K.E.; Choi, Y.W.; Kim, S.T.; Kim, Y.K. Components of rhizome extract of Cnidium officinale Makino and their in vitro biological effects. Molecules 2011, 16, 8833–8847. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, K.H. Purification and identification of falcarinol, polyacetylene family, from Glehnia littoralis capable of cytotoxicity on jurkat T lymphoma. Cancer Prev. Res. 2008, 13, 216–221. [Google Scholar]
- Nakano, Y.; Matsunaga, H.; Saita, T.; Mori, M.; Katano, M.; Okabe, H. Antiproliferative constituents in Umbelliferae plants II. Screening for polyacetylenes in some Umbelliferae plants, and isolation of panaxynol and falcarindiol from the root of Heracleum moellendorffii. Biol. Pharm. Bull. 1998, 21, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokosuka, A.; Tatsuno, S.; Komine, T.; Mimaki, Y. Chemical constituents of the roots and rhizomes of Saposhnikovia divaricata and their cytotoxic activity. Nat. Prod. Commun. 2017, 12, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Cruz, J.; Kim, D.H.; Hwang, S.G. Anticancer effects of Cnidium officinale Makino extract mediated through apoptosis and cell cycle arrest in the HT-29 human colorectal cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 5117–5121. [Google Scholar] [PubMed]
- Zheng, X.; Zheng, X.; Zhang, C.; Zhang, Q.; Jiang, Y.; Tu, P. Cytotoxic polyacetylenes isolated from the roots and rhizomes of Notopterygium incisum. Chin. Chem. Lett. 2019, 30, 428–430. [Google Scholar] [CrossRef]
- Hu, C.Q.; Chang, J.J.; Lee, K.H. Antitumor agents, 115. Seselidiol, a new cytotoxic polyacetylene from Seseli mairei. J. Nat. Prod. 1990, 53, 932–935. [Google Scholar] [CrossRef]
- Mi, C.N.; Wang, H.; Chen, H.Q.; Cai, C.H.; Li, S.P.; Mei, W.L.; Dai, H.F. Polyacetylenes from the roots of Swietenia macrophylla King. Molecules 2019, 24, 1291. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; Di, Y.T.; Li, S.F.; Geng, Z.L.; He, H.P.; Wang, Y.H.; Wang, Y.Y.; Li, Y.; Li, S.L.; Ha, X.J. Polyynes from Toona ciliata var. ciliata and related cytotoxic activity. Helv. Chim. Acta 2011, 94, 376–381. [Google Scholar] [CrossRef]
- Parwati, N.W.M.; Lindayani, I.K.; Ratnawati, R.; Winarsih, S.; Nurseta, T. Possible effect of tea plant parasite, Scurrula atropurpurea (Blume) Danser, on growth inhibition of culture HeLa cells in vitro through DNA repair and apoptosis intrinsic pathways mechanism. Asian Pac. J. Trop. Dis. 2015, 5, 743–746. [Google Scholar] [CrossRef]
- Ohashi, K.; Winarno, H.; Mukai, M.; Inoue, M.; Prana, M.S.; Simanjuntak, P.; Shibuya, H. Indonesian medicinal plants. XXV. Cancer cell invasion inhibitory effects of chemical constituents in the parasitic plant Scurrula atropurpurea (Loranthaceae). Chem. Pharm. Bull. (Tokyo) 2003, 51, 343–345. [Google Scholar] [CrossRef] [Green Version]
- Marles, R.J.; Farnsworth, N.R.; Neill, D.A. Isolation of a novel cytotoxic polyacetylene from a traditional anthelmintic medicinal plant, Minquartia guianensis. J. Nat. Prod. 1989, 52, 261–266. [Google Scholar] [CrossRef]
- Fort, D.M.; King, S.R.; Carlson, T.J.; Nelson, S.T. Minquartynoic acid from Coula edulis. Biochem. Syst. Ecol. 2000, 28, 489–490. [Google Scholar] [CrossRef]
- Ito, A.; Cui, B.; Chavez, D.; Chai, H.B.; Shin, Y.G.; Kawanishi, K.; Kardono, L.B.; Riswan, S.; Farnsworth, N.R.; Cordell, G.A.; et al. Cytotoxic polyacetylenes from the twigs of Ochanostachys amentacea. J. Nat. Prod. 2001, 64, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Cardellina, J.H., 2nd; Boyd, M.R. Absolute stereochemistry and anti-HIV activity of minquartynoic acid, a polyacetylene from Ochanostachys amentacea. Nat. Prod. Lett. 2001, 15, 21–26. [Google Scholar] [CrossRef]
- Li, C.; Lee, D.; Graf, T.N.; Phifer, S.S.; Nakanishi, Y.; Riswan, S.; Setyowati, F.M.; Saribi, A.M.; Soejarto, D.D.; Farnsworth, N.R.; et al. Bioactive constituents of the stem bark of Mitrephora glabra. J. Nat. Prod. 2009, 72, 1949–1953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, C.; He, Z.H.; Huang, J.; Du, X.; Wang, L.; Wei, S.P.; Pu, L.; Wang, Q. Highly enantioselective synthesis and anticancer activities of chiral conjugated diynols. Chembiochem 2018, 19, 2293–2299. [Google Scholar] [CrossRef]
- Listunov, D.; Saffon-Merceron, N.; Joly, E.; Fabing, I.; Génisson, Y.; Maraval, V.; Chauvin, R. Ethynylogation approach in pharmacophore design: From alkynyl-to butadiynyl-carbinols vs. antitumoral cytotoxicity. Tetrahedron 2016, 72, 6697–6704. [Google Scholar] [CrossRef]
- El Arfaoui, D.; Listunov, D.; Fabing, I.; Oukessou, M.; Frongia, C.; Lobjois, V.; Samson, A.; Ausseil, F.; Ben-Tama, A.; El Hadrami, E.M.; et al. Identification of chiral alkenyl- and alkynylcarbinols as pharmacophores for potent cytotoxicity. Chemmedchem. 2013, 8, 1779–1786. [Google Scholar] [CrossRef]
- de Castro Barbosa, M.L.; da Conceicao, R.A.; Fraga, A.G.M.; Camarinha, B.D.; de Carvalho Silva, G.C.; Lima, A.G.F.; Cardoso, E.A.; de Oliveira Freitas Lione, V. NF-κB signaling pathway inhibitors as anticancer drug candidates. Anticancer Agents Med. Chem. 2017, 17, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 2001, 107, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Murakami, T.; Kageura, T.; Ninomiya, K.; Toguchida, I.; Nishida, N.; Yoshikawa, M. Hepatoprotective and nitric oxide production inhibitory activities of coumarin and polyacetylene constituents from the roots of Angelica furcijuga. Bioorg. Med. Chem. Lett. 1998, 8, 2191–2196. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Nishida, N.; Ninomiya, K.; Ohgushi, T.; Kubo, M.; Morikawa, T.; Matsuda, H. Inhibitory effects of coumarin and acetylene constituents from the roots of Angelica furcijuga on D-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2006, 14, 456–463. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, P.; Son, D.; Kim, H.; Kim, S.Y. Falcarindiol inhibits nitric oxide-mediated neuronal death in lipopolysaccharide-treated organotypic hippocampal cultures. Neuroreport 2003, 14, 1941–1944. [Google Scholar] [CrossRef]
- Shim, S.Y.; Lee, S.; Kim, M.; Lee, J.W.; Hwang, B.Y.; Lee, M. Falcarindiol from Angelica koreana down-regulated IL-8 and up-regulated IL-10 in colon epithelial cells. Nat. Prod. Sci. 2017, 23, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.M.; Lundgaard, N.H.; Light, M.E.; Stafford, G.I.; van Staden, J.; Jäger, A.K. The polyacetylene falcarindiol with COX-1 activity isolated from Aegopodium podagraria L. J. Ethnopharmacol. 2007, 113, 176–178. [Google Scholar] [CrossRef]
- Dang, N.H.; Zhang, X.F.; Zheng, M.S.; Son, K.H.; Chang, H.W.; Kim, H.P.; Bae, K.H.; Kang, S.S. Inhibitory constituents against cyclooxygenases from Aralia cordata Thunb. Arch. Pharm. Res. 2005, 28, 28–33. [Google Scholar] [CrossRef]
- Liu, J.H.; Zschocke, S.; Bauer, R. A polyacetylenic acetate and a coumarin from Angelica pubescens f. biserrata. Phytochemistry 1998, 49, 211–213. [Google Scholar] [CrossRef]
- Alanko, J.; Kurahashi, Y.; Yoshimoto, T.; Yamamoto, S.; Baba, K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem. Pharm. 1994, 48, 1979–1981. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 1. Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase/cyclooxygenase. Phytother. Res. 2005, 19, 81–102. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 2. Medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists. Phytother Res. 2005, 19, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Teng, C.M.; Lee, J.C.; Ko, F.N.; Chen, S.C.; Wu, T.S. Antiplatelet components in Panax ginseng. Planta Med. 1990, 56, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.M.; Kuo, S.C.; Ko, F.N.; Lee, J.C.; Lee, L.G.; Chen, S.C.; Huang, T.F. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim. Biophys. Acta 1989, 990, 315–320. [Google Scholar] [CrossRef]
- Appendino, G.; Tagliapietra, S.; Nano, G.M.; Picci, V. An antiplatelet acetylene from the leaves of Ferula communis. Fitoterapia 1993, 64, 179. [Google Scholar]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- He, P.; Yang, C.; Ye, G.; Xie, H.; Zhong, W. Risks of colorectal neoplasms and cardiovascular thromboembolic events after the combined use of selective COX-2 inhibitors and aspirin with 5-year follow-up: A meta-analysis. Colorectal Dis. 2019, 21, 417–426. [Google Scholar] [CrossRef]
- Crosby, D.G.; Aharonson, N. The structure of carotatoxin, a natural toxicant from carrot. Tetrahedron 1967, 23, 465–472. [Google Scholar] [CrossRef]
- Uwai, K.; Ohashi, K.; Takaya, Y.; Ohta, T.; Tadano, T.; Kisara, K.; Shibusawa, K.; Sakakibara, R.; Oshima, Y. Exploring the structural basis of neurotoxicity in C17-polyacetylenes isolated from water hemlock. J. Med. Chem. 2000, 43, 4508–4515. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Christensen, L.P.; Vach, W.; Ritskes-Hoitinga, J.; Brandt, K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J. Agric. Food Chem. 2005, 53, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Raju, J. Azoxymethane-induced rat aberrant crypt foci: Relevance in studying chemoprevention of colon cancer. World J. Gastroenterol. 2008, 14, 6632–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, R.P. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef]
- Corpet, D.E.; Pierre, F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon cancer chemoprevention in rats, mice and humans. Eur. J. Cancer 2005, 41, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008, 118, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARγ as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Fajas, L.; Egler, V.; Reiter, R.; Miard, S.; Lefebvre, A.M.; Auwerx, J. PPARγ controls cell proliferation and apoptosis in an RB-dependent manner. Oncogene 2003, 22, 4186–4193. [Google Scholar] [CrossRef] [Green Version]
- Resetar, M.; Liu, X.; Herdlinger, S.; Kunert, O.; Pferschy-Wenzig, E.M.; Latkolik, S.; Steinacher, T.; Schuster, D.; Bauer, R.; Dirsch, V.M. Polyacetylenes from Oplopanax horridus and Panax ginseng: Relationship between structure and PPARγ activation. J. Nat. Prod. 2020, 83, 918–926. [Google Scholar] [CrossRef] [Green Version]
- Kobaek-Larsen, M.; Nielsen, D.S.; Kot, W.; Krych, Ł.; Christensen, L.P.; Baatrup, G. Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. Bmc Res. Notes 2018, 11, 411. [Google Scholar] [CrossRef]
- Le, H.T.; Nguyen, H.T.; Min, H.Y.; Hyun, S.Y.; Kwon, S.; Lee, Y.; Le, T.H.V.; Lee, J.; Park, J.H.; Lee, H.Y. Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett. 2018, 412, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Burns, T.F. Targeting heat shock proteins in cancer: A promising therapeutic approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudesco, C.; Cause, S.; Jego, G.; Garrido, C. Hsp70: A cancer target inside and outside the cell. Methods Mol. Biol. 2018, 1709, 371–396. [Google Scholar] [CrossRef]
- Katano, M.; Yamamoto, H.; Matsunaga, H.; Mori, M.; Takata, K.; Nakamura, M. Cell growth inhibitory substance isolated from Panax ginseng root: Panaxytriol. Gan Kagaku Ryoho 1990, 17, 1045–1049. [Google Scholar]
- Yun, H.; Chou, T.C.; Dong, H.; Tian, Y.; Li, Y.M.; Danishefsky, S.J. Total synthesis as a resource in drug discovery: The first in vivo evaluation of panaxytriol and its derivatives. J. Org. Chem. 2005, 70, 10375–10380. [Google Scholar] [CrossRef]










| Falcarindiol (μg/mL) | Falcarinol (μg/mL) | |||
|---|---|---|---|---|
| 0 | 1 | 5 | 10 | |
| 0 | 1.0 | 1.01 ± 0.25 | 0.36 ± 0.25 | 0.18 ± 0.01 |
| 1 | 1.35 ± 0.36 | 0.71 ± 0.08 b | 0.58 ± 0.04 | 0.22 ± 0.08 |
| 5 | 1.03 ± 0.32 | 0.53 ± 0.06 b | nd c | nd |
| 10 | 0.52 ± 0.17 | 0.17 ± 0.03 b | nd | nd |
| Acetylenic Oxylipin | IC50 (μM) | ||
|---|---|---|---|
| MCF-7 | H1299 | HepG2 | |
| 1 | 43.1 ± 0.1 a | 30.8 ± 0.1 | 45.2 ± 0.2 |
| 4 | > 100 | > 100 | > 100 |
| 5 | 29.4 ± 1.0 | 22.1 ± 0.9 | 23.6 ± 2.0 |
| 7 | 19.0 ± 0.9 | 16.4 ± 0.7 | 15.9 ± 0.7 |
| 8 | 29.6 ± 1.9 | 21.3 ± 1.9 | 11.7 ± 1.2 |
| 9 | 67.8 ± 2.3 | 37.6 ± 1.3 | 22.7 ± 0.2 |
| 19 | 45.6 ± 1.5 | 14.6 ± 0.8 | 20.8 ± 1.2 |
| 55 | 31.7 ± 1.3 | 24.9 ± 0.9 | 35.3 ± 0.5 |
| 56 | 1.3 ± 0.6 | 0.6 ± 0.2 | 1.4 ± 0.7 |
| 57 | 13.5 ± 1.9 | 12.8 ± 0.9 | 24.9 ± 0.6 |
| 58 | 7.3 ± 0.4 | 10.7 ± 0.8 | 19.2 ± 2.2 |
| 62 | 15.1 ± 1.9 | 12.1 ± 0.9 | 23.6 ± 2.0 |
| 95 | 85.7 ± 0.4 | 31.9 ± 0.2 | 54.2 ± 1.6 |
| 97 | > 100 | > 100 | 29.7 ± 2.7 |
| 104 | 66.7 ± 1.2 | 36.0 ± 1.6 | 47.6 ± 1.9 |
| Taxol | 0.0022 ± 0.0003 | 0.0018 ± 0.0008 | 0.0020 ± 0.0007 |
| Size of Neoplasms | µg Falcarindiol and Falcarinol (1:1)/g Feed | |||||
|---|---|---|---|---|---|---|
| 0 (n = 20) | 0.16 (n = 20) | 0.48 (n = 20) | 1.4 (n = 20) | 7 (n = 20) | 35 (n = 20) | |
| Mean ACF < 7 crypts a | 205 ± 36 | 207 ± 28 | 180 ± 29 | 171 ± 26 | 150 ± 31 | 145 ± 19 |
| Mean ACF > 7 crypts b | nd c | 14 ± 3.7 | 12 ± 4.1 | 10 ± 3.7 | nd | 8 ± 3.5 |
| Total number of macroscopic polyp neoplasms d | 21 | 18 | 19 | 13 | 12 | 7 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. https://doi.org/10.3390/molecules25112568
Christensen LP. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules. 2020; 25(11):2568. https://doi.org/10.3390/molecules25112568
Chicago/Turabian StyleChristensen, Lars Porskjær. 2020. "Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development" Molecules 25, no. 11: 2568. https://doi.org/10.3390/molecules25112568
APA StyleChristensen, L. P. (2020). Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules, 25(11), 2568. https://doi.org/10.3390/molecules25112568