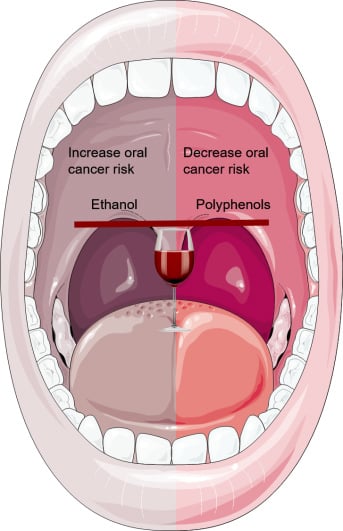
Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus
Abstract
:1. Introduction
2. Wine as Oral Cavity Cancer-Enhancer
2.1. Formation and Accumulation of Acetaldehyde in Oral Cavity after Wine Ingestion
2.2. Ethanol/Acetaldehyde Genotoxicity
2.3. Ethanol/Acetaldehyde and Pre-Cancerous Lesions
3. Wine as Oral Cavity Cancer-Preventer
3.1. In Vitro Studies
3.2. In Vivo Studies
3.3. Human Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global cancer observatory: Cancer today. Lyon Fr. Int. Agency Res. Cancer 2018, 144, 1941–1953. [Google Scholar]
- Radoi, L.; Luce, D. A review of risk factors for oral cavity cancer: The importance of a standardized case definition. Community Dent. Oral Epidemiol. 2013, 41, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lewin, F.; Norell, S.E.; Johansson, H.; Gustavsson, P.; Wennerberg, J.; Biorklund, A.; Rutqvist, L.E. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer 1998, 82, 1367–1375. [Google Scholar] [CrossRef]
- Moreno-Lopez, L.A.; Esparza-Gomez, G.C.; Gonzalez-Navarro, A.; Cerero-Lapiedra, R.; Gonzalez-Hernandez, M.J.; Dominguez-Rojas, V. Risk of oral cancer associated with tobacco smoking, alcohol consumption and oral hygiene: A case-control study in Madrid, Spain. Oral Oncol. 2000, 36, 170–174. [Google Scholar] [CrossRef]
- Radoi, L.; Paget-Bailly, S.; Cyr, D.; Papadopoulos, A.; Guida, F.; Schmaus, A.; Cenee, S.; Menvielle, G.; Carton, M.; Lapotre-Ledoux, B.; et al. Tobacco smoking, alcohol drinking and risk of oral cavity cancer by subsite: Results of a French population-based case-control study, the ICARE study. Eur. J. Cancer Prev. 2013, 22, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Andre, K.; Schraub, S.; Mercier, M.; Bontemps, P. Role of alcohol and tobacco in the aetiology of head and neck cancer: A case-control study in the Doubs region of France. Eur. J. Cancer B Oral Oncol. 1995, 31, 301–309. [Google Scholar] [CrossRef]
- Brugere, J.; Guenel, P.; Leclerc, A.; Rodriguez, J. Differential effects of tobacco and alcohol in cancer of the larynx, pharynx, and mouth. Cancer 1986, 57, 391–395. [Google Scholar] [CrossRef]
- Franceschi, S.; Talamini, R.; Barra, S.; Baron, A.E.; Negri, E.; Bidoli, E.; Serraino, D.; La Vecchia, C. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990, 50, 6502–6507. [Google Scholar]
- Mashberg, A.; Boffetta, P.; Winkelman, R.; Garfinkel, L. Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer 1993, 72, 1369–1375. [Google Scholar] [CrossRef]
- Merletti, F.; Boffetta, P.; Ciccone, G.; Mashberg, A.; Terracini, B. Role of tobacco and alcoholic beverages in the etiology of cancer of the oral cavity/oropharynx in Torino, Italy. Cancer Res. 1989, 49, 4919–4924. [Google Scholar]
- Tuyns, A.J.; Esteve, J.; Raymond, L.; Berrino, F.; Benhamou, E.; Blanchet, F.; Boffetta, P.; Crosignani, P.; Del Moral, A.; Lehmann, W.; et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int. J. Cancer 1988, 41, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Maasland, D.H.; Brandt, P.A.V.D.; Kremer, B.; Goldbohm, R.A.; Schouten, L.J. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer 2014, 14, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, D.I.; Purkayastha, M.; Chestnutt, I.G. The changing epidemiology of oral cancer: Definitions, trends, and risk factors. Br. Dent. J. 2018, 225, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Maliakal, P.; Lu, H.; Lee, M.-J.; Yang, C.S. Urinary and Plasma Levels of Resveratrol and Quercetin in Humans, Mice, and Rats after Ingestion of Pure Compounds and Grape Juice. J. Agric. Food Chem. 2004, 52, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano-Ferrer, E.; Urpi, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharm. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Monakhova, Y.B. Short-term salivary acetaldehyde increase due to direct exposure to alcoholic beverages as an additional cancer risk factor beyond ethanol metabolism. J. Exp. Clin. Cancer Res. 2011, 30, 3. [Google Scholar] [CrossRef] [Green Version]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Mccloskey, L.P.; Mahaney, P. An Enzymatic Assay for Acetaldehyde in Grape Juice and Wine. Am. J. Enol. Vitic. 1981, 32, 159–162. [Google Scholar]
- Jackowetz, J.; De Orduña, R.M. Survey of SO2 binding carbonyls in 237 red and white table wines. Food Control. 2013, 32, 687–692. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Sohnius, E.M. The role of acetaldehyde outside ethanol metabolism in the carcinogenicity of alcoholic beverages: Evidence from a large chemical survey. Food Chem. Toxicol. 2008, 46, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Paiano, V.; Bianchi, G.; Davoli, E.; Negri, E.; Fanelli, R.; Fattore, E. Risk assessment for the Italian population of acetaldehyde in alcoholic and non-alcoholic beverages. Food Chem. 2014, 154, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Linderborg, K.; Joly, J.P.; Visapää, J.-P.; Salaspuro, M. Potential mechanism for Calvados-related oesophageal cancer. Food Chem. Toxicol. 2008, 46, 476–479. [Google Scholar] [CrossRef]
- Han, G.; Webb, M.R.; Waterhouse, A.L. Acetaldehyde reactions during wine bottle storage. Food Chem. 2019, 290, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kanteres, F.; Lachenmeier, D.W.; Rehm, J. Alcohol in Mayan Guatemala: Consumption, distribution, production and composition of cuxa. Addiction 2009, 104, 752–759. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Sohnius, E.M.; Attig, R.; Lopez, M.G. Quantification of selected volatile constituents and anions in Mexican Agave spirits (Tequila, Mezcal, Sotol, Bacanora). J. Agric. Food Chem. 2006, 54, 3911–3915. [Google Scholar] [CrossRef]
- Oliveira, V.A.; Vicente, M.A.; Fietto, L.G.; Castro, I.M.D.; Coutrim, M.X.; Schüller, D.; Alves, H.; Casal, M.; Santos, J.D.O.; Araújo, L.D.; et al. Biochemical and Molecular Characterization of Saccharomyces cerevisiae Strains Obtained from Sugar-Cane Juice Fermentations and Their Impact in Cachaça Production. Appl. Environ. Microbiol. 2008, 74, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Salaspuro, M. Acetaldehyde as a common denominator and cumulative carcinogen in digestive tract cancers. Scand. J. Gastroenterol. 2009, 44, 912–925. [Google Scholar] [CrossRef]
- Salaspuro, M. Acetaldehyde and gastric cancer. J. Dig. Dis. 2011, 12, 51–59. [Google Scholar] [CrossRef]
- Gaonkar, P.P.; Patankar, S.R.; Tripathi, N.; Sridharan, G. Oral bacterial flora and oral cancer: The possible link. J. Oral Maxillofac. Pathol. 2018, 22, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Cirauqui, M.L.G.; Nieminen, M.; Novak-Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.-D.; Rautemaa, R. Production of carcinogenic acetaldehyde byCandida albicansfrom patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2012, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; Kolev, S.D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Hitomi, Y.; Ohtsu, A.; Shimada, H.; Kashiwase, Y.; Sasaki, H.; Yoshida, S.; Esumi, H. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int. J. Cancer 2000, 88, 342–350. [Google Scholar] [CrossRef]
- Moritani, K.; Takeshita, T.; Shibata, Y.; Ninomiya, T.; Kiyohara, Y.; Yamashita, Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015, 21, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Tsutsumi, E.; Imazeki, H.; Suwa, Y.; Nakamura, C.; Mizukami, T.; Yokoyama, T. Salivary Acetaldehyde Concentration According to Alcoholic Beverage Consumed and Aldehyde Dehydrogenase-2 Genotype. Alcohol. Clin. Exp. Res. 2008, 32, 1607–1614. [Google Scholar] [CrossRef]
- Homann, N.; Jousimies-Somer, H.; Jokelainen, K.; Heine, R.; Salaspuro, M. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef]
- Vondracek, M.; Xi, Z.; Larsson, P.; Baker, V.; Mace, K.; Pfeifer, A.; Tjalve, H.; Donato, M.T.; Gomez-Lechon, M.J.; Grafstrom, R.C. Cytochrome P450 expression and related metabolism in human buccal mucosa. Carcinogenesis 2001, 22, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Haiyarit, P.; Ma, N.; Hiraku, Y.; Pinlaor, S.; Yongvanit, P.; Jintakanon, D.; Murata, M.; Oikawa, S.; Kawanishi, S. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Sci. 2005, 96, 553–559. [Google Scholar] [CrossRef]
- Ma, N.; Tagawa, T.; Hiraku, Y.; Murata, M.; Ding, X.; Kawanishi, S. 8-Nitroguanine formation in oral leukoplakia, a premalignant lesion. Nitric Oxide 2006, 14, 137–143. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Boil. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.H.; Beevi, S.S.; Geetha, A. Enhanced lipid peroxidation and nitric oxide products with deranged antioxidant status in patients with head and neck squamous cell carcinoma. Oral Oncol. 2007, 43, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Korde, S.D.; Basak, A.; Chaudhary, M.; Goyal, M.; Vagga, A. Enhanced Nitrosative and Oxidative Stress with Decreased Total Antioxidant Capacity in Patients with Oral Precancer and Oral Squamous Cell Carcinoma. Oncology 2011, 80, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.S.; Rasheed, A.M.H.; Geetha, A. Evaluation of Oxidative Stress and Nitric Oxide Levels in Patients with Oral Cavity Cancer. Jpn. J. Clin. Oncol. 2004, 34, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Warnakulasuriya, S.; Parkkila, S.; Nagao, T.; Preedy, V.R.; Pasanen, M.; Koivisto, H.; Niemelä, O. Demonstration of ethanol-induced protein adducts in oral leukoplakia (pre-cancer) and cancer. J. Oral Pathol. Med. 2007, 37, 157–165. [Google Scholar] [CrossRef]
- Ogden, G.R.; Wight, A. Aetiology of oral cancer: Alcohol. Br. J. Oral Maxillofac. Surg. 1998, 36, 247–251. [Google Scholar] [CrossRef]
- Wight, A.; Ogden, G.R. Possible mechanisms by which alcohol may influence the development of oral cancer—a review. Oral Oncol. 1998, 34, 441–447. [Google Scholar] [CrossRef]
- Imanowski, U.A.; Stickel, F.; Maier, H.; Gärtner, U.; Seitz, H.K. Effect of alcohol on gastrointestinal cell regeneration as a possible mechanism in alcohol-associated carcinogenesis. Alcohol 1995, 12, 111–115. [Google Scholar] [CrossRef]
- Theruvathu, J.A.; Jaruga, P.; Nath, R.G.; Dizdaroglu, M.; Brooks, P.J. Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 2005, 33, 3513–3520. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Hosoya, M.; Shimoyama, T.; Takahashi, S.; Zhang, J.F.; Tsutsumi, E.; Suzuki, Y.; Suwa, Y.; Nakayama, T. Catalytic removal of acetaldehyde in saliva by a Gluconobacter strain. J. Biosci. Bioeng. 2012, 114, 268–274. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; De Monte, S.M. Acetaldehyde Adducts in Alcoholic Liver Disease. Oxidative Med. Cell. Longev. 2010, 3, 406524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Mcintee, E.J.; Cheng, G.; Shi, Y.; Villalta, P.W.; Hecht, S.S. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 2000, 13, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-S.; Oyama, T.; Matsuda, T.; Isse, T.; Yamaguchi, T.; Tanaka, M.; Tsuji, M.; Kawamoto, T. The effect of ethanol on the formation of N 2-ethylidene-dG adducts in mice: Implications for alcohol-related carcinogenicity of the oral cavity and esophagus. Biomarkers 2012, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Meng, L.; Bliss, R.L.; Jensen, J.A.; Hatsukami, R.K.; Hecht, S.S. Kinetics of DNA adduct formation in the oral cavity after drinking alcohol. Cancer Epidemiol. Biomark. Prev. 2012, 21, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbo, S.; Meng, L.; Bliss, R.L.; Jensen, J.A.; Hatsukami, D.K.; Hecht, S.S. Time course of DNA adduct formation in peripheral blood granulocytes and lymphocytes after drinking alcohol. Mutagenesis 2012, 27, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boy, S.C. Leukoplakia and erythroplakia of the oral mucosa-a brief overview. Clin. Rev. 2012, 67, 558–560. [Google Scholar]
- Feller, L.; Lemmer, J. Oral Squamous Cell Carcinoma: Epidemiology, Clinical Presentation and Treatment. J. Cancer Ther. 2012, 3, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Mahomed, F. Oral submucous fibrosis - a potentially malignant condition of growing concern. Clin. Rev. 2012, 67, 562–565. [Google Scholar]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma overview. Oral Oncol. 2009, 45, 301–308. [Google Scholar] [CrossRef]
- Van Der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef]
- Fukuda, M.; Kusama, K.; Sakashita, H. Molecular insights into the proliferation and progression mechanisms of the oral cancer: Strategies for the effective and personalized therapy. Jpn. Dent. Sci. Rev. 2012, 48, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Roepman, P.; A Wessels, L.F.; Kettelarij, N.; Kemmeren, P.; Miles, A.J.; Lijnzaad, P.; Tilanus, M.G.J.; Koole, R.; Hordijk, G.-J.; Van Der Vliet, P.C.; et al. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nat. Genet. 2005, 37, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Macigo, F.G.; Mwaniki, D.L.; Guthua, S.W. The association between oral leukoplakia and use of tobacco, alcohol and that based on relative risks assessment in Kenya. Eur. J. Oral Sci. 1995, 103, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Ko, Y.-C.; Huang, H.-L.; Chao, Y.-Y.; Tsai, C.-C.; Shieh, T.-Y.; Lin, L.-M. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br. J. Cancer 2003, 88, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petti, S.; Scully, C. Association between different alcoholic beverages and leukoplakia among non- to moderate-drinking adults: A matched case–control study. Eur. J. Cancer 2006, 42, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.E.; Psoter, W.J.; Cleveland, D.; Cohen, N.; Mohit-Tabatabai, M.; Kosis, D.L.; Eisenberg, E. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control. 2007, 18, 919–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaber, M.A.; Porter, S.; Gilthorpe, M.; Bedi, R.; Scully, C. Risk factors for oral epithelial dysplasia—the role of smoking and alcohol. Oral Oncol. 1999, 35, 151–156. [Google Scholar] [CrossRef]
- Kulasegaram, R.; Downer, M.; Jullien, J.; Zakrzewska, J.M.; Speight, P. Case-control study of oral dysplasia and risk habits among patients of a dental hospital. Oral Oncol. 1995, 31, 227–231. [Google Scholar] [CrossRef]
- E Morse, D.; Katz, R.V.; Pendrys, D.G.; Holford, T.R.; Krutchkoff, D.J.; Eisenberg, E.; Kosis, D.; Mayne, S.T. Smoking and drinking in relation to oral epithelial dysplasia. Cancer Epidemiol. Biomark. Prev. 1996, 5, 769–777. [Google Scholar]
- Li, L.; Psoter, W.J.; Buxó, C.J.; Elias, A.; Cuadrado, L.; Morse, D.E. Smoking and drinking in relation to oral potentially malignant disorders in Puerto Rico: A case-control study. BMC Cancer 2011, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Purdue, M.P.; Hashibe, M.; Berthiller, J.; La Vecchia, C.; Maso, L.D.; Herrero, R.; Franceschi, S.; Castellsagué, X.; Wei, Q.; Sturgis, E.M.; et al. Type of Alcoholic Beverage and Risk of Head and Neck Cancer—A Pooled Analysis Within the INHANCE Consortium. Am. J. Epidemiol. 2008, 169, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Introduction to Phenolics. In Understanding Wine Chemistry; Wiley: Hoboken, NJ, USA, 2016; pp. 99–104. [Google Scholar]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a biological fluid: History, production, and role in disease prevention. J. Clin. Lab. Anal. 1997, 11, 287–313. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health–New Evidence. Eur. J. Clin. Nutr. 2018, 72, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Sánchez-Carballido, M.A.; Suárez, C.D.; Gómez-Mora, J.A.; Bonneau, N. Effects of flavonoids on tongue squamous cell carcinoma. Cell Boil. Int. 2020, 44, 686–720. [Google Scholar] [CrossRef] [PubMed]
- Belobrov, S.; Seers, C.; Reynolds, E.; Cirillo, N.; McCullough, M. Functional and molecular effects of a green tea constituent on oral cancer cells. J. Oral Pathol. Med. 2019, 48, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzui, M.; Weinstein, I.B. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin. Cancer Res. 2001, 7, 4220–4229. [Google Scholar] [PubMed]
- Elattar, T.M.; Virji, A.S. Effect of tea polyphenols on growth of oral squamous carcinoma cells in vitro. Anticancer. Res. 2000, 20, 3459–3465. [Google Scholar] [PubMed]
- López, E.P.-F.; Wang, Q.-T.; Wei, W.; Jornet, P.L. Potential chemotherapeutic effects of diosgenin, zoledronic acid and epigallocatechin-3-gallate on PE/CA-PJ15 oral squamous cancer cell line. Arch. Oral Boil. 2017, 82, 141–146. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yoshida, H.; Matsuda, S.; Ryoke, T.; Ohta, K.; Ohmori, M.; Yamamoto, S.; Kiyoshima, T.; Kobayashi, M.; Sano, K. The therapeutic potential of epigallocatechin-3-gallate against human oral squamous cell carcinoma through inhibition of cell proliferation and induction of apoptosis: In vitro and in vivo murine xenograft study. Mol. Med. Rep. 2019, 20, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Gu, K.; Wang, Q.; Chen, X.; Fu, X.; Wang, Y.; Wen, Y. Epigallocatechin-3-gallate affects the proliferation, apoptosis, migration and invasion of tongue squamous cell carcinoma through the hippo-TAZ signaling pathway. Int. J. Mol. Med. 2018, 42, 2615–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, K.C.; Gunasekaran, P.; Varalakshmi, E.; Hara, Y.; Nagini, S. In vitro evaluation of the anticancer effect of lactoferrin and tea polyphenol combination on oral carcinoma cells. Cell Boil. Int. 2007, 31, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ramshankar, V.; Krishnamurthy, A. Chemoprevention of oral cancer: Green tea experience. J. Nat. Sci. Boil. Med. 2014, 5, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.-H.; Horng, C.-T.; Lee, C.-F.; Chiang, N.-N.; Tsai, F.-J.; Lu, C.-C.; Chiang, J.-H.; Hsu, Y.-M.; Yang, J.-S.; Chen, F.-A. Epigallocatechin gallate sensitizes cisplatin-resistant oral cancer CAR cell apoptosis and autophagy through stimulating AKT/STAT3 pathway and suppressing multidrug resistance 1 signaling. Environ. Toxicol. 2016, 32, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Amin, A.R.; Shin, D.M. Chemoprevention of head and neck cancer with green tea polyphenols. Cancer Prev. Res. 2010, 3, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, U.-L.; Choi, S.-W. The Chemopreventive Properties and Therapeutic Modulation of Green Tea Polyphenols in Oral Squamous Cell Carcinoma. Isrn Oncol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.-Y.; Lien, C.-H.; Lee, M.-F.; Huang, C.-Y. Quercetin suppresses cellular migration and invasion in human head and neck squamous cell carcinoma (HNSCC). Biomedicine 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Walle, T. Quercetin Induces Necrosis and Apoptosis in SCC-9 Oral Cancer Cells. Nutr. Cancer 2005, 53, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Wang, H.; Hu, Z.; Huang, Y.; Yao, F.; Sun, S.; Wu, B. Quercetin Inhibits Proliferation and Drug Resistance in KB/VCR Oral Cancer Cells and Enhances Its Sensitivity to Vincristine. Nutr. Cancer 2014, 67, 126–136. [Google Scholar] [CrossRef]
- Chen, S.F.; Nieh, S.; Jao, S.W.; Liu, C.L.; Wu, C.H.; Chang, Y.C.; Yang, C.Y.; Lin, Y.S. Quercetin suppresses drug-resistant spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.W.; Kim, J.H.; Song, K.; Kim, S.H.; Yoon, J.-H.; Kim, K.-S. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother. Res. 2009, 24, S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-kappaB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Ma, Y.; Yao, C.; Liu, H.; Yu, F.; Lin, J.; Lu, K.; Liao, C.; Chueh, F.; Chung, J. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol. Lett. 2018, 15, 9663–9672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Fang, Z.; Zha, Z.; Sun, Q.; Wang, H.; Sun, M.; Qiao, B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur. J. Pharm. 2019, 847, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chan, C.-Y.; Chou, I.-T.; Lien, C.-H.; Hung, H.-C.; Lee, M.-F. Quercetin induces growth arrest through activation of FOXO1 transcription factor in EGFR-overexpressing oral cancer cells. J. Nutr. Biochem. 2013, 24, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS ONE 2012, 7, e46404. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Gao, J.; Cheng, X.; Hou, C.; Yang, Y.; Qiu, Y.; Xu, M.; Zhang, Y.; Huang, S. Grape seed proanthocyanidins inhibit the proliferation, migration and invasion of tongue squamous cell carcinoma cells through suppressing the protein kinase B/nuclear factor-kappaB signaling pathway. Int. J. Mol. Med. 2017, 40, 1881–1888. [Google Scholar] [CrossRef]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef]
- Qi, C.; Li, S.; Jia, Y.; Wang, L. Blueberry anthocyanins induce G2/M cell cycle arrest and apoptosis of oral cancer KB cells through down-regulation methylation of p53. Yi Chuan Hered. 2014, 36, 566–573. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry Extracts Inhibit Growth and Stimulate Apoptosis of Human Cancer Cells In Vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total Cranberry Extract versus Its Phytochemical Constituents: Antiproliferative and Synergistic Effects against Human Tumor Cell Lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ding, H.; Casto, B.; Stoner, G.D.; D’Ambrosio, S.M. Inhibition of the Growth of Premalignant and Malignant Human Oral Cell Lines by Extracts and Components of Black Raspberries. Nutr. Cancer 2005, 51, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, K.A.; Rawal, Y.; Renner, R.J.; Schwartz, S.J.; Tian, Q.; Larsen, P.E.; Mallery, S.R. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutr. Cancer 2006, 54, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-J.; Wang, I.-C.; Hsiao, Y.-T.; Lin, H.-Y.; Tang, N.-Y.; Hung, T.-C.; Quan, C.; Lien, J.-C.; Chung, J. Anthocyanins from Black Rice (Oryza sativa L.) Demonstrate Antimetastatic Properties by Reducing MMPs and NF-?B Expressions in Human Oral Cancer CAL 27 Cells. Nutr. Cancer 2015, 67, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Yue, E.; Tuguzbaeva, G.; Chen, X.; Qin, Y.; Li, A.; Sun, X.; Dong, C.; Liu, Y.; Yu, Y.; Zahra, S.M.; et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine 2019, 56, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.R.; Stoner, G.D.; Larsen, P.E.; Fields, H.W.; Rodrigo, K.A.; Schwartz, S.J.; Tian, Q.; Dai, J.; Mumper, R.J. Formulation and In-Vitro and In-Vivo Evaluation of a Mucoadhesive Gel Containing Freeze Dried Black Raspberries: Implications for Oral Cancer Chemoprevention. Pharm. Res. 2007, 24, 728–737. [Google Scholar] [CrossRef]
- Vervandier-Fasseur, D.; Latruffe, N. The Potential Use of Resveratrol for Cancer Prevention. Molecules 2019, 24, 4506. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Song, W.; Li, D.; Cai, L.; Zhao, Y. Resveratrol As A Natural Regulator Of Autophagy For Prevention And Treatment Of Cancer. Onco Targets Ther. 2019, 12, 8601–8609. [Google Scholar] [CrossRef] [Green Version]
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; De Filippis, A.; Ravagnan, G.; De Maria, S.; Muzio, L.L. Resveratrol (3,5,4′-trihydroxystilbene) and its properties in oral diseases. Exp. Ther. Med. 2017, 14, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Kim, H.-J.; Lee, M.-H.; Yu, S.-K.; Kim, C.S.; Kook, J.-K.; Chun, H.S.; Park, E.; Lee, S.-Y.; Kim, S.-G.; et al. Resveratrol induces apoptosis of KB human oral cancer cells. J. Korean Soc. Appl. Boil. Chem. 2011, 54, 966–971. [Google Scholar] [CrossRef]
- Yu, X.D.; Yang, J.L.; Zhang, W.L.; Liu, D.X. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumour Biol. 2016, 37, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Gu, M.; Takahata, T.; Frederick, B.; Agarwal, C.; Siriwardana, S.; Agarwal, R.; Sclafani, R. Resveratrol selectively induces DNA Damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin. Cancer Res. 2011, 17, 5402–5411. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.-Y.; Hsieh, Y.-H.; Yang, S.-F.; Chen, C.-T.; Tang, C.-H.; Chou, M.-Y.; Chuang, Y.-T.; Lin, C.-W.; Chen, M.-K. Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells. J. Oral Pathol. Med. 2014, 44, 699–706. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, G.; Xiang, W.; Pei-Jun, W.; Bin, Z. Effects of resveratrol on oral squamous cell carcinoma (OSCC) cells in vitro. J. Cancer Res. Aclin. Oncol. 2014, 140, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Ko, H.-A.; Shieh, T.-M.; Chi, T.-C.; Chen, H.-I.; Chen, Y.-T.; Yu, Y.-H.; Yang, S.-H.; Chang, S.-S. Advanced glycation end products influence oral cancer cell survival via Bcl-xl and Nrf-2 regulation in vitro. Oncol. Lett. 2017, 13, 3328–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, A.; Narayanan, S.; Balasubramanian, G.; Sethuraman, S.; Krishnan, U.M. Dual drug loaded nanoliposomal chemotherapy: A promising strategy for treatment of head and neck squamous cell carcinoma. Eur. J. Pharm. Biopharm. 2016, 99, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Wu, C.-Y.; Chin, Y.-T.; Li, Z.-L.; Pan, Y.-S.; Huang, T.-Y.; Su, P.-Y.; Lee, S.-Y.; Crawford, D.R.; Su, K.-W.; et al. NDAT suppresses pro-inflammatory gene expression to enhance resveratrol-induced anti-proliferation in oral cancer cells. Food Chem. Toxicol. 2019, 136, 111092. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chin, Y.-T.; Shih, Y.-J.; Chen, Y.-R.; Chung, Y.-Y.; Lin, C.-Y.; Hsiung, C.-N.; Whang-Peng, J.; Lee, S.-Y.; Lin, H.-Y.; et al. Resveratrol antagonizes thyroid hormone-induced expression of checkpoint and proliferative genes in oral cancer cells. J. Dent. Sci. 2019, 14, 255–262. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Chen, Y.-S.; Chin, Y.-T.; Li, Z.-L.; Shih, Y.-J.; Yang, Y.-C.S.; Changou, C.A.; Su, P.-Y.; Wang, S.-H.; Wu, Y.-H.; et al. Thyroid hormone-induced expression of inflammatory cytokines interfere with resveratrol-induced anti-proliferation of oral cancer cells. Food Chem. Toxicol. 2019, 132, 110693. [Google Scholar] [CrossRef]
- Hayashi, F.; Kasamatsu, A.; Endo-Sakamoto, Y.; Eizuka, K.; Hiroshima, K.; Kita, A.; Saito, T.; Koike, K.; Tanzawa, H.; Uzawa, K. Increased expression of tripartite motif (TRIM) like 2 promotes tumoral growth in human oral cancer. Biochem. Biophys. Res. Commun. 2019, 508, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Elattar, T.M.; Virji, A.S. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anti-Cancer Drugs 1999, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Horng, C.; Hsieh, M.; Chen, H.; Huang, Y.; Yang, J.; Wang, G.; Chiang, J.; Chen, H.; Lu, C.; et al. Autophagy and apoptotic machinery caused by Polygonum cuspidatum extract in cisplatin-resistant human oral cancer CAR cells. Oncol. Rep. 2019, 41, 2549–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.-J.; Chin, M.-C.; Lin, C.-C.; His, Y.-T.; Lo, Y.-S.; Chuang, Y.-C.; Chen, M.-K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. 2018, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- De Moura, C.F.G.; Soares, G.R.; Ribeiro, F.A.P.; Silva, M.J.D.; Vilegas, W.; Santamarina, A.B.; Pisani, L.P.; Estadella, D.; Ribeiro, D.A. Evaluation of the Chemopreventive Activity of Grape Skin Extract Using Medium-term Oral Carcinogenesis Assay Induced by 4-Nitroquinoline 1-Oxide. Anticancer. Res. 2018, 39, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Sabitha, K.E.; Shyamaladevi, C.S. Therapeutic efficacy of green tea polyphenols on cellular thiols in 4-Nitroquinoline 1-oxide-induced oral carcinogenesis. Chem. Interact. 2004, 149, 81–87. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, G.; Dai, J.; Sun, Y.U.; Hoffman, R.M.; Yang, Z.; Fan, Y. Chemoprevention by Quercetin of Oral Squamous Cell Carcinoma by Suppression of the NF-kappaB Signaling Pathway in DMBA-treated Hamsters. Anticancer Res. 2017, 37, 4041–4049. [Google Scholar]
- Casto, B.C.; Kresty, L.A.; Kraly, C.L.; Pearl, D.K.; Knobloch, T.J.; A Schut, H.; Stoner, G.D.; Mallery, S.R.; Weghorst, C. Chemoprevention of oral cancer by black raspberries. Anticancer. Res. 2003, 22, 4005–4015. [Google Scholar]
- Chen, K.-M.; Sun, Y.-W.; Kawasawa, Y.I.; Salzberg, A.C.; Zhu, J.; Gowda, K.; Aliaga, C.; Amin, S.; Atkins, H.; El-Bayoumy, K. Black Raspberry Inhibits Oral Tumors in Mice Treated with the Tobacco Smoke Constituent Dibenzo(def,p)chrysene Via Genetic and Epigenetic Alterations. Cancer Prev. Res. 2020, 13, 357–366. [Google Scholar] [CrossRef]
- Berta, G.N.; Salamone, P.; Sprio, A.E.; Di Scipio, F.; Marinos, L.M.; Sapino, S.; Carlotti, M.E.; Cavalli, R.; Di Carlo, F. Chemoprevention of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch by topical application of resveratrol complexed with 2-hydroxypropyl-β-cyclodextrin. Oral Oncol. 2010, 46, 42–48. [Google Scholar] [CrossRef]
- Zheng, T.; Feng, H.; Liu, L.; Peng, J.; Xiao, H.; Yu, T.; Zhou, Z.; Li, Y.; Zhang, Y.; Bai, X.; et al. Enhanced antiproliferative effect of resveratrol in head and neck squamous cell carcinoma using GE11 peptide conjugated liposome. Int. J. Mol. Med. 2019, 43, 1635–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, A.S.; Liu, D.; Martin, J.; Tang, X.-M.; Lee, J.J.; El-Naggar, A.K.; Wistuba, I.; Culotta, K.S.; Mao, L.; Gillenwater, A.; et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev. Res. 2009, 2, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Sun, Z.; Han, C.; Chen, J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc. Soc. Exp. Boil. Med. 1999, 220, 218–224. [Google Scholar]
- Yoon, A.J.; Shen, J.; Santella, R.M.; Philipone, E.M.; Wu, H.-C.; Eisig, S.B.; Blitzer, A.; Close, L.G.; Zegarelli, D.J. Topical Application of Green Tea Polyphenol (−)-Epigallocatechin-3-gallate (EGCG) for Prevention of Recurrent Oral Neoplastic Lesions. J. Orofac. Sci. 2012, 4, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shumway, B.S.; Kresty, L.A.; Larsen, P.E.; Zwick, J.C.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D.; Mallery, S.R. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin. Cancer Res. 2008, 14, 2421–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallery, S.R.; Zwick, J.C.; Pei, P.; Tong, M.; Larsen, P.E.; Shumway, B.S.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008, 68, 4945–4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knobloch, T.J.; Uhrig, L.K.; Pearl, D.K.; Casto, B.C.; Warner, B.M.; Clinton, S.K.; Sardo-Molmenti, C.L.; Ferguson, J.M.; Daly, B.T.; Riedl, K.M.; et al. Suppression of pro-inflammatory and pro-survival biomarkers in oral cancer patients consuming a black raspberry phytochemical-rich troche. Cancer Prev. Res. 2015, 9, 159–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, A.S.; Zanuy, M.; Ángeles, V.; Cano, M.A.; Alonso, A.R.; Bravo, I.A.; Blanco, E.R.; Jiménez, M.M.; Sanz, M.L. Adherence to Mediterranean diet: A comparison of patients with head and neck cancer and healthy population. Endocrinol. Diabetes Y Nutr. 2019, 66, 417–424. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.; Latruffe, N.; de Gaetano, G. Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus. Molecules 2020, 25, 2569. https://doi.org/10.3390/molecules25112569
Silva P, Latruffe N, de Gaetano G. Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus. Molecules. 2020; 25(11):2569. https://doi.org/10.3390/molecules25112569
Chicago/Turabian StyleSilva, Paula, Norbert Latruffe, and Giovanni de Gaetano. 2020. "Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus" Molecules 25, no. 11: 2569. https://doi.org/10.3390/molecules25112569
APA StyleSilva, P., Latruffe, N., & de Gaetano, G. (2020). Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus. Molecules, 25(11), 2569. https://doi.org/10.3390/molecules25112569