Protective Effects of Some Grapevine Polyphenols against Naturally Occurring Neuronal Death
Abstract
:1. Introduction
2. Results
2.1. Effect of Ethanolic Media onto Naturally Occurring Neuronal Death (NOND) in Postnatal Cerebellum
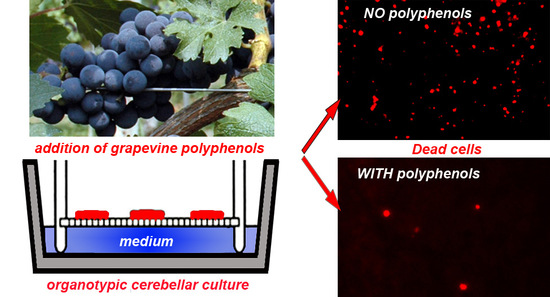
2.2. Effects of PPs onto NOND and Ethanol-Induced Cell Death
2.3. Comparison of the Effectiveness of PPs in Counteracting NOND
3. Discussion
3.1. Suitability of the Ex Vivo Approach to Study the Neuroprotective Effects of Grapevine PPs
3.2. The Relationship Between PPs Chemical Structure and Neuroprotective Effects
3.3. Clues for In Vivo Neuroprotection
4. Materials and Methods
4.1. PPs
4.2. Animals
4.3. Preparation of Cerebellar Cultures
4.4. Preparation of PPs-Containing Media, Incubation of Cultures with PPs and Staining of Dead Cells
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [Green Version]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. PTR 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Ingolfsson, H.I.; Thakur, P.; Herold, K.F.; Hobart, E.A.; Ramsey, N.B.; Periole, X.; de Jong, D.H.; Zwama, M.; Yilmaz, D.; Hall, K.; et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 2014, 9, 1788–1798. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Visioli, F. The resveratrol fiasco. Pharmacol. Res. 2014, 90, 87. [Google Scholar] [CrossRef]
- Kelly, E.; Vyas, P.; Weber, J.T. Biochemical Properties and Neuroprotective Effects of Compounds in Various Species of Berries. Molecules 2017, 23, 26. [Google Scholar] [CrossRef] [Green Version]
- Kedrina-Okutan, O.; Novello, V.; Hoffmann, T.; Hadersdorfer, J.; Occhipinti, A.; Schwab, W.; Ferrandino, A. Constitutive Polyphenols in Blades and Veins of Grapevine (Vitis vinifera L.) Healthy Leaves. J. Agric. Food Chem. 2018, 66, 10977–10990. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Laurie, V.F.; Waterhouse, A.L. A simple method to separate red wine nonpolymeric and polymeric phenols by solid-phase extraction. J. Agric. Food Chem. 2006, 54, 2839–2844. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharm. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Dang, W. Chapter 17—The Controversy Around Sirtuins and Their Functions in Aging. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 227–241. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [Green Version]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [Green Version]
- Beher, D.; Wu, J.; Cumine, S.; Kim, K.W.; Lu, S.C.; Atangan, L.; Wang, M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 2009, 74, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, R.F.; Garcia-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Wine, resveratrol and health: A review. Nat. Prod. Commun. 2009, 4, 635–658. [Google Scholar] [CrossRef] [Green Version]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2010, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Ding, Y.; Li, X.; Kong, D.; Xin, C.; Yang, X.; Zhang, C.; Rong, Z.; Yao, C.; Lu, S.; et al. Polydatin protects SH-SY5Y in models of Parkinson’s disease by promoting Atg5-mediated but parkin-independent autophagy. Neurochem. Int. 2020, 134, 104671. [Google Scholar] [CrossRef] [PubMed]
- Potdar, S.; Parmar, M.S.; Ray, S.D.; Cavanaugh, J.E. Protective effects of the resveratrol analog piceid in dopaminergic SH-SY5Y cells. Arch. Toxicol. 2018, 92, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohanaki, H.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Pelargonidin improves memory deficit in amyloid beta25–35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016, 83, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Ross, E.K.; Khatter, S.; Miller, K.; Linseman, D.A. Chemical basis for the disparate neuroprotective effects of the anthocyanins, callistephin and kuromanin, against nitrosative stress. Free Radic. Biol. Med. 2017, 103, 23–34. [Google Scholar] [CrossRef]
- Matias, A.A.; Rosado-Ramos, R.; Nunes, S.L.; Figueira, I.; Serra, A.T.; Bronze, M.R.; Santos, C.N.; Duarte, C.M. Protective Effect of a (Poly) phenol-Rich Extract Derived from Sweet Cherries Culls against Oxidative Cell Damage. Molecules 2016, 21, 406. [Google Scholar] [CrossRef]
- Ereminas, G.; Majiene, D.; Sidlauskas, K.; Jakstas, V.; Ivanauskas, L.; Vaitiekaitis, G.; Liobikas, J. Neuroprotective properties of anthocyanidin glycosides against H2O2-induced glial cell death are modulated by their different stability and antioxidant activity in vitro. Biomed. Pharmacother. 2017, 94, 188–196. [Google Scholar] [CrossRef]
- Ferrandino, A.; Carra, A.; Rolle, L.; Schneider, A.; Schubert, A. Profiling of hydroxycinnamoyl tartrates and acylated anthocyanins in the skin of 34 Vitis vinifera genotypes. J. Agric. Food Chem. 2012, 60, 4931–4945. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Cui, Y.; Zhang, S.; Sun, B. Separation and purification of polyphenols from red wine extracts using high speed counter current chromatography. J. Chromatogr. Banalytical Technol. Biomed. Life Sci. 2017, 1054, 105–113. [Google Scholar] [CrossRef]
- Rocha-Parra, D.; Chirife, J.; Zamora, C.; de Pascual-Teresa, S. Chemical Characterization of an Encapsulated Red Wine Powder and Its Effects on Neuronal Cells. Molecules 2018, 23, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimidt, H.L.; Garcia, A.; Martins, A.; Mello-Carpes, P.B.; Carpes, F.P. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model. Food Res. Int. 2017, 100 Part 1, 442–448. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Gaidin, S.G.; Mal’tseva, V.N.; Zinchenko, V.P.; Turovsky, E.A. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol. Cell. Neurosci. 2019, 96, 10–24. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Moran, C.; Scotto di Palumbo, A.; Bramham, J.; Moran, A.; Rooney, B.; De Vito, G.; Egan, B. Effects of a Six-Month Multi-Ingredient Nutrition Supplement Intervention of Omega-3 Polyunsaturated Fatty Acids, vitamin D, Resveratrol, and Whey Protein on Cognitive Function in Older Adults: A Randomised, Double-Blind, Controlled Trial. J. Prev. Alzheimer’s Dis. 2018, 5, 175–183. [Google Scholar]
- Levin, J.; Maass, S.; Schuberth, M.; Giese, A.; Oertel, W.H.; Poewe, W.; Trenkwalder, C.; Wenning, G.K.; Mansmann, U.; Sudmeyer, M.; et al. Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019, 18, 724–735. [Google Scholar] [CrossRef]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimer’s Dement. (N. Y.) 2019, 5, 184–196. [Google Scholar] [CrossRef]
- Lossi, L.; Merighi, A. In vivo cellular and molecular mechanisms of neuronal apoptosis in the mammalian CNS. Progr. Neurobiol. 2003, 69, 287–312. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantazis, N.J.; West, J.R.; Dai, D. The nitric oxide-cyclic GMP pathway plays an essential role in both promoting cell survival of cerebellar granule cells in culture and protecting the cells against ethanol neurotoxicity. J. Neurochem. 1998, 70, 1826–1838. [Google Scholar] [CrossRef] [PubMed]
- Kouzoukas, D.E.; Bhalla, R.C.; Pantazis, N.J. Activation of cyclic GMP-dependent protein kinase blocks alcohol-mediated cell death and calcium disruption in cerebellar granule neurons. Neurosci. Lett. 2018, 676, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.M.; Miller, M.W. Ethanol-induced neuronal death in organotypic cultures of rat cerebral cortex. Dev. Brain Res. 2003, 147, 135–141. [Google Scholar] [CrossRef]
- Lossi, L.; Cocito, C.; Alasia, S.; Merighi, A. Ex vivo imaging of active caspase 3 by a FRET-based molecular probe demonstrates the cellular dynamics and localization of the protease in cerebellar granule cells and its regulation by the apoptosis-inhibiting protein survivin. Mol. Neurodegener. 2016, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Bertelli, A.; Falchi, M.; Lo Scalzo, R.; Morelli, R. EPR evaluation of the antiradical activity of wines containing high concentrations of resveratrol. Drugs Exp. Clin. Res. 2004, 30, 111–115. [Google Scholar]
- Piano, F.; Bertolone, E.; Pes, D.; Asproudi, A.; Borsa, D. Focusing on bioactive compounds in grapes: Stilbenes in Uvalino cv. Eur. Food Res. Technol. 2013, 237, 897–904. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal Phenolic Phytochemicals in Selected California Wines and Their Antioxidant Activity in Inhibiting Oxidation of Human Low-Density Lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Pantelić, M.M.; Dabić Zagorac, D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. J. Arb. Fur Pharm. Verfahr. E.V 2018, 127, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Akoh, C.C.; Fischer, J.; Krewer, G. Absorption of anthocyanins from blueberry extracts by caco-2 human intestinal cell monolayers. J. Agric. Food Chem. 2006, 54, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef]
- Kilmartin, P.A. Electrochemical detection of natural antioxidants: Principles and protocols. Antioxid. Redox Signal. 2001, 3, 941–955. [Google Scholar] [CrossRef] [Green Version]
- Vo, Q.V.; Cam Nam, P.; Bay, M.V.; Minh Thong, N.; Hieu, L.T.; Mechler, A. A theoretical study of the radical scavenging activity of natural stilbenes. RSC Adv. 2019, 9, 42020–42028. [Google Scholar] [CrossRef] [Green Version]
- Bastianetto, S.; Krantic, S.; Chabot, J.G.; Quirion, R. Possible involvement of programmed cell death pathways in the neuroprotective action of polyphenols. Curr. Alzheimer Res. 2011, 8, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Piao, X.-L.; Kim, H.Y.; Cho, E.-J.; Baek, S.-H.; Kwon, S.W.; Park, J.H. Resveratrol oligomers from Vitis amurensis attenuate beta-amyloid-induced oxidative stress in PC12 cells. Boil. Pharm. Bull. 2007, 30, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Mol. 2018, 23, 2328. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement. Altern. Med. 2014, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Cai, Z.; Cai, M.; Liu, K.; Liu, D.; Zhang, Q.; Tan, J.; Ma, Q. AMPK/SIRT1/p38 MAPK signaling pathway regulates alcohol induced neurodegeneration by resveratrol. Mol. Med. Rep. 2018, 17, 5402–5408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, D.-Q.; Liao, Z.; Wang, B.; Gong, S.; Wang, C.; Zhang, M.-Z.; Wang, G.-H.; Cai, H.; Liao, F.-F.; et al. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol. Neurodegener. 2015, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Nakanishi, T.; Shimoda, H.; Nakamura, S.; Tsuruma, K.; Shimazawa, M.; Matsuda, H.; Yoshikawa, M.; Hara, H. Purple rice extract and its constituents suppress endoplasmic reticulum stress-induced retinal damage in vitro and in vivo. Life Sci. 2013, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Ullah, I.; Lee, H.Y.; Kim, M.O. Anthocyanins Protect Against Ethanol-Induced Neuronal Apoptosis via GABAB1 Receptors Intracellular Signaling in Prenatal Rat Hippocampal Neurons. Mol. Neurobiol. 2013, 48, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3β Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2018, 56, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Yoon, G.H.; Kim, M.O. Protection of the Developing Brain with Anthocyanins Against Ethanol-Induced Oxidative Stress and Neurodegeneration. Mol. Neurobiol. 2014, 51, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-N.; Chi, C.-W.; Lin, Y.-L.; Chen, C.-F.; Shiao, Y.-J. The Neuroprotective Effects of Phytoestrogens on Amyloid β Protein-induced Toxicity Are Mediated by Abrogating the Activation of Caspase Cascade in Rat Cortical Neurons. J. Boil. Chem. 2000, 276, 5287–5295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Yang, M.; Qi, X.; Shen, X.; Chen, X.; Zhang, F. Quercetin ameliorates ischemia/reperfusion-induced cognitive deficits by inhibiting ASK1/JNK3/caspase-3 by enhancing the Akt signaling pathway. Biochem. Biophys. Res. Commun. 2016, 478, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sarkar, S.; Mandal, A.K.; Das, N. Neuroprotective Role of Nanoencapsulated Quercetin in Combating Ischemia-Reperfusion Induced Neuronal Damage in Young and Aged Rats. PLoS ONE 2013, 8, e57735. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kang, S.U.; Pyun, J.; Lee, M.H.; Hwang, H.S.; Lee, H. Epicatechin protects auditory cells against cisplatin-induced death. Apoptosis 2008, 13, 1184–1194. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.; Zhong, X.; Yao, W.; Xiao, Q.; Wen, Q.; Yang, B.; Wei, Q. Epigallocatechin Gallate Reduces Amyloid β-Induced Neurotoxicity via Inhibiting Endoplasmic Reticulum Stress-Mediated Apoptosis. Mol. Nutr. Food Res. 2018, 62, 1700890. [Google Scholar] [CrossRef]
- Nam, Y.J.; Lee, D.H.; Shin, Y.K.; Sohn, D.S.; Lee, C.S. Flavanonol Taxifolin Attenuates Proteasome Inhibition-Induced Apoptosis in Differentiated PC12 Cells by Suppressing Cell Death Process. Neurochem. Res. 2014, 40, 480–491. [Google Scholar] [CrossRef]
- Bastianetto, S.; Menard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lossi, L.; Mioletti, S.; Aimar, P.; Bruno, R.; Merighi, A. In vivo analysis of cell proliferation and apoptosis in the CNS. In Cellular and Molecular Methods in Neuroscience Research; Merighi, A., Carmignoto, G., Eds.; Springer: New York, NY, USA, 2002; pp. 235–258. [Google Scholar]






| PPs | Density of Dead Cells (PI+ cells/mm2) | Ratio (b/a) | |
|---|---|---|---|
| Control (a) | PP (b) | (c) | |
| Pn-3OG | 57.43 | 16.2 | 0.28 |
| (+)-Catechin | 31.32 | 9.15 | 0.29 |
| Taxifolin | 4.128 | 1.249 | 0.30 |
| Q-3OG | 34.04 | 10.53 | 0.31 |
| Resveratrol | 34.04 | 13.59 | 0.40 |
| Polydatin | 6.78 | 3.298 | 0.49 |
| Mv-3OG (Water) | 17.56 | 9.41 | 0.54 |
| PPs | Type of study | Organ/tissue/cell/Species | Death inductor | Ref |
| Resveratrol | In vitro | PC12 cells/Rat | Aβ | [64] |
| Primary cortical neurons/Rat | Aβ | [65] | ||
| 661W photoreceptor cells/Mouse | Blue light | [66] | ||
| SH-SY5Y cells/human | Ethanol | [67] | ||
| In vivo | Brain/Rat | Ethanol | [67] | |
| Polydatin | In vitro | PC12 cells/Rat | Aβ | [64] |
| In vivo | Primary cortical neurons/rat | Aβ | [65] | |
| Rat models of Parkinson’s disease | Rotenone | [68] | ||
| Anthocyanins mix) | In vitro | Hippocampal HT22 cells/Mouse | Aβ | [69] |
| RGC-5/Mouse | H2O2 or Tunicamycin | [70] | ||
| Primary hippocampal neurons/Rat | Ethanol | [71] | ||
| In vivo | APP/PS1 mouse model of AD | N/A | [69] | |
| Brain/Rat | LPS | [72] | ||
| Hippocampus/Rat | Ethanol | [73] | ||
| Peonidin | In vitro | RGC-5/Mouse | Tunicamycin | [70] |
| Malvidin | In vitro | 661W photoreceptor cells/mouse | Blue light | [66] |
| Quercetin | In vitro | Primary cortical neurons/Rat | Aβ | [74] |
| Primary hippocampal neurons/Rat | OGD | [75] | ||
| In vivo | Brain/Rat | IRI | [75] | |
| Hippocampus/Mouse | IRI | [76] | ||
| Brain/Rat | IRI | [77] | ||
| Epicatechin | In vivo | Brain/Rat | LPS | [78] |
| In vitro | Auditory cells | Cisplatin | [79] | |
| Epigallocatechin | In vitro | SH-SY5Y cells/human | Aβ | [80] |
| In vivo | APP/PS1 mouse model of AD | Tunicamycin or Tapsigargin | [80] | |
| Taxifolin | In vitro | PC12 cells/Rat | Proteasome inhibition | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lossi, L.; Merighi, A.; Novello, V.; Ferrandino, A. Protective Effects of Some Grapevine Polyphenols against Naturally Occurring Neuronal Death. Molecules 2020, 25, 2925. https://doi.org/10.3390/molecules25122925
Lossi L, Merighi A, Novello V, Ferrandino A. Protective Effects of Some Grapevine Polyphenols against Naturally Occurring Neuronal Death. Molecules. 2020; 25(12):2925. https://doi.org/10.3390/molecules25122925
Chicago/Turabian StyleLossi, Laura, Adalberto Merighi, Vittorino Novello, and Alessandra Ferrandino. 2020. "Protective Effects of Some Grapevine Polyphenols against Naturally Occurring Neuronal Death" Molecules 25, no. 12: 2925. https://doi.org/10.3390/molecules25122925
APA StyleLossi, L., Merighi, A., Novello, V., & Ferrandino, A. (2020). Protective Effects of Some Grapevine Polyphenols against Naturally Occurring Neuronal Death. Molecules, 25(12), 2925. https://doi.org/10.3390/molecules25122925