Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Thermal Stability of the Polymers and Copolymers
2.2. Phase Behaviour
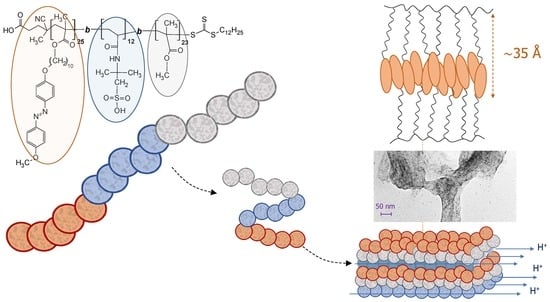
2.3. Phase Structure
2.4. Light-Responsive Behaviour
2.5. Conductivity Response
3. Materials and Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Collings, P.; Hird, M. Introduction to Liquid Crystals. Chemistry and Physics; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Goodby, J.W.; Mandle, R.J.; Davis, E.J.; Zhong, T.; Cowling, S.J. What makes a liquid crystal? the effect of free volume on soft matter. Liq. Cryst. 2015, 42, 593–622. [Google Scholar] [CrossRef]
- Reinitzer, F. Beiträge zur kenntniss des cholesterins. Monatsh. Chem. Verw. Teile. Anderer. Wiss. 1888, 9, 421–441. [Google Scholar] [CrossRef]
- Kato, T.; Kamikawa, Y. Hydrogen-bonded systems: Discrete defined aggregates by intermolecular H−bonding, amides, carboxylic acids, and heterocycles. In Handbook of Liquid Crystals, 2nd ed.; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Schadt, M. Liquid crystal materials and liquid crystal displays. Annu. Rev. Mater. Sci. 1997, 27, 305–379. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, S.; Sadashiva, B.; Suresh, K. Liquid-crystals of disc-like molecules. Pramana 1977, 9, 471–480. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Wang, L.; Urbas, A.M.; Li, Q. Nature-inspired emerging chiral liquid crystal nanostructures: From molecular self-assembly to DNA mesophase and nanocolloids. Adv. Mater. 2018. [Google Scholar] [CrossRef]
- Sage, I. Thermochromic liquid crystals. Liq. Cryst. 2011, 38, 1551–1561. [Google Scholar] [CrossRef]
- Castillo-Valles, M.; Martinez-Bueno, A.; Gimenez, R.; Sierra, T.; Ros, M.B. Beyond liquid crystals: New research trends for mesogenic molecules in liquids. J. Mater. Chem. C 2019, 7, 14454–14470. [Google Scholar] [CrossRef]
- Shen, Y.; Dierking, I. Perspectives in liquid-crystal-aided nanotechnology and nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Zhao, Y. Photothermally driven liquid crystal polymer actuators. Mater. Chem. Front. 2018, 2, 1932–1943. [Google Scholar] [CrossRef]
- de Haan, L.T.; Gimenez-Pinto, V.; Konya, A.; Thanh-Son, N.; Verjans, J.M.N.; Sanchez-Somolinos, C.; Selinger, J.V.; Selinger, R.L.B.; Broer, D.J.; Schenning, A.P.H.J. Accordion-like actuators of multiple 3D patterned liquid crystal polymer films. Adv. Funct. Mater. 2014, 24, 1251–1258. [Google Scholar] [CrossRef]
- Hird, M. Ferroelectricity in liquid crystals—Materials, properties and applications. Liq. Cryst. 2011, 38, 1467–1493. [Google Scholar] [CrossRef]
- Martinez-Felipe, A. Liquid crystal polymers and ionomers for membrane applications. Liq. Cryst. 2011, 38, 1607–1626. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, X.; Kuang, T.; Chang, L.; Fu, D.; Yang, J.; Fan, P.; Zhong, M. Polyelectrolyte/mesoporous silica hybrid materials for the high performance multiple-detection of pH value and temperature. Polym. Chem. 2015, 6, 3529–3536. [Google Scholar] [CrossRef]
- van Kuringen, H.P.C.; Eikelboom, G.M.; Shishmanova, I.K.; Broer, D.J.; Schenning, A.P.H.J. Responsive nanoporous smectic liquid crystal polymer networks as efficient and selective adsorbents. Adv. Funct. Mater. 2014, 24, 5045–5051. [Google Scholar] [CrossRef]
- Pang, X.; Lv, J.; Zhu, C.; Qi, L.; Yu, Y. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv. Mater. 2019, 1904224. [Google Scholar] [CrossRef]
- Sharma, A.; Neshat, A.; Mahnen, C.J.; Nielsen, A.D.; Snyder, J.; Stankovich, T.L.; Daum, B.G.; LaSpina, E.M.; Beltrano, G.; Gao, Y.; et al. Biocompatible, biodegradable and porous liquid crystal elastomer scaffolds for spatial cell cultures. Macromol. Biosci. 2015, 15, 200–214. [Google Scholar] [CrossRef]
- Safinya, C.R.; Ewert, K.K.; Leal, C. Cationic liposome-nucleic acid complexes: Liquid crystal phases with applications in gene therapy. Liq. Cryst. 2011, 38, 1715–1723. [Google Scholar] [CrossRef] [Green Version]
- Ciferri, A. A supramolecular polymerisation model for the structurisation of DNA-lipid complexes. Liq. Cryst. 2012, 39, 1231–1236. [Google Scholar] [CrossRef]
- Bushby, R.J.; Kawata, K. Liquid crystals that affected the world: Discotic liquid crystals. Liq. Cryst. 2011, 38, 1415–1426. [Google Scholar] [CrossRef]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Soberats, B.; Yoshio, M.; Ichikawa, T.; Taguchi, S.; Ohno, H.; Kato, T. 3D anhydrous proton-transporting nanochannels formed by self-assembly of liquid crystals composed of a sulfobetaine and a sulfonic acid. J. Am. Chem. Soc. 2013, 135, 15286–15289. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, K.A.; Moore, R.B. State of understanding of nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Felipe, A.; Imrie, C.T.; Ribes-Greus, A. Spectroscopic and thermal characterization of the swelling behavior of nafion membranes in mixtures of water and methanol. J. Appl. Polym. Sci. 2013, 127, 246–256. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Moliner-Estopinan, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly(vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, 1000–1011. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Schuster, M.; Meyer, W. Anhydrous proton-conducting polymers. Annu. Rev. Mater. Res. 2003, 33, 233–261. [Google Scholar] [CrossRef]
- Cho, B. Nanostructured organic electrolytes. RSC Adv. 2014, 4, 395–405. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Norder, B.; Garcia, S.J.; Picken, S.J.; Madsen, L.A.; Dingemans, T.J. Water and sodium transport and liquid crystalline alignment in a sulfonated aramid membrane. J. Membr. Sci. 2015, 489, 194–203. [Google Scholar] [CrossRef]
- Montane, X.; Vilasrao Bhosale, S.; Antonio Reina, J.; Giamberini, M. Columnar liquid crystalline polyglycidol derivatives: A novel alternative for proton-conducting membranes. Polymer 2015, 66, 100–109. [Google Scholar] [CrossRef]
- Stoeva, Z.; Lu, Z.; Ingram, M.D.; Imrie, C.T. A new polymer electrolyte based on a discotic liquid crystal triblock copolymer. Electrochim. Acta 2013, 93, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Guo, X.; Aili, D.; Pan, C.; Li, Q.; Fang, J. Poly(imide benzimidazole)s for high temperature polymer electrolyte membrane fuel cells. J. Membr. Sci. 2014, 454, 351–358. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Yang, G.; Zhan, S. New anhydrous proton exchange membranes based on fluoropolymers blend imidazolium poly (aromatic ether ketonejs for high temperature polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2018, 43, 8464–8473. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Vanti, L.; Mohd Alauddin, S.; Zaton, D.; Aripin, N.F.K.; Giacinti-Baschetti, M.; Imrie, C.T.; Ribes-Greus, A.; Martinez-Felipe, A. Ionically conducting and photoresponsive liquid crystalline terpolymers: Towards multifunctional polymer electrolytes. Eur. Polym. J. 2018, 109, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Felipe, A.; Badia, J.D.; Santonja-Blasco, L.; Imrie, C.T.; Ribes-Greus, A. A kinetic study of the formation of smectic phases in novel liquid crystal ionogens. Eur. Polym. J. 2013, 49, 1553–1563. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Felipe, A.; Imrie, C.T.; Ribes-Greus, A. Study of structure formation in side-chain liquid crystal copolymers by variable temperature fourier transform infrared spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8714–8721. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Lu, Z.; Henderson, P.A.; Picken, S.J.; Norder, B.; Imrie, C.T.; Ribes-Greus, A. Synthesis and characterisation of side chain liquid crystal copolymers containing sulfonic acid groups. Polymer 2012, 53, 2604–2612. [Google Scholar] [CrossRef]
- Aguilar, M.; Gallardo, A.; Fernandez, M.; San Roman, J. In situ quantitative H-1 NMR monitoring of monomer consumption: A simple and fast way of estimating reactivity ratios. Macromolecules 2002, 35, 2036–2041. [Google Scholar] [CrossRef]
- Mishra, A.; Choudhary, V. Synthesis, characterizations and thermal behavior of methyl methacrylate and N-(p-carboxyphenyl) methacrylamide/arylamide copolymers. J. Appl. Polym. Sci. 2000, 78, 259–267. [Google Scholar] [CrossRef]
- Talpur, M.; Oracz, P.; Kaim, A. Study of methyl methacrylate-acrylamide copolymerization system in cyclohexanone in the absence of conventional radical initiator. Polymer 1996, 37, 4149–4154. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Kuan, W.; Remy, R.; Mackay, M.E.; Epps Thomas, H. Controlled ionic conductivity via tapered block polymer electrolytes. RSC Adv. 2015, 5, 12597–12604. [Google Scholar] [CrossRef] [Green Version]
- Young, W.-S.; Epps, T.H., III. Ionic conductivities of block copolymer electrolytes with various conducting pathways: Sample preparation and processing considerations. Macromolecules 2012, 45, 4689–4697. [Google Scholar] [CrossRef]
- Choi, U.H.; Lee, M.; Wang, S.; Liu, W.; Winey, K.I.; Gibson, H.W.; Colby, R.H. Ionic conduction and dielectric response of poly(imidazolium acrylate) ionomers. Macromolecules 2012, 45, 3974–3985. [Google Scholar] [CrossRef]
- Byun, I.; Lee, J.; Jeong, K.; Han, Y. Synthesis of high X block copolymers with LC moieties and PMMA segments using RAFT polymerization, and their nanostructure morphologies. Polymer 2018, 145, 184–193. [Google Scholar] [CrossRef]
- Carrasco-Hemandez, S.; Gutierrez, J.; Tercjak, A. Optical reversible behavior of poly(ethylene-b-ethylene oxide) block copolymer dispersed liquid crystal blends. Eur. Polym. J. 2017, 91, 187–196. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Y.; Hu, Z.; Chen, Z.; Hu, J.; Yang, L. pH responsive self-assembly and drug release behavior of aliphatic liquid crystal block polycarbonate with pendant cholesteryl groups. J. Mol. Liq. 2018, 266, 405–412. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. [Google Scholar] [CrossRef]
- Alauddin, S.M.; Aripin, N.F.K.; Velayutham, T.S.; Chaganava, I.; Martinez-Felipe, A. The role of conductivity and molecular mobility on the photoanisotropic response of a new azo-polymer containing sulfonic groups. J. Photochem. Photobiol. A Chem. 2020, 389, 112268. [Google Scholar] [CrossRef]
- Scott, A.J.; Duever, T.A.; Penlidis, A. The role of pH, ionic strength and monomer concentration on the terpolymerization of 2-acrylamido-2-methylpropane sulfonic acid, acrylamide and acrylic acid. Polymer 2019, 177, 214–230. [Google Scholar] [CrossRef]
- Bouhamed, H.; Boufi, S.; Magnin, A. Dispersion of alumina suspension using comb-like and diblock copolymers produced by RAFT polymerization of AMPS and MPEG. J. Colloid Interface Sci. 2007, 312, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Zhang, Q.; Tian, N. Synthesis and properties of gel polymer electrolyte membranes based on novel comb-like methyl methacrylate copolymers. J. Membr. Sci. 2010, 349, 279–286. [Google Scholar] [CrossRef]
- Imrie, C.T.; Schleeh, T.; Karasz, F.E.; Attard, G.S. Dependence of the transitional properties of polystyrene-based side-chain liquid-crystalline polymers on the chemical nature of the mesogenic group. Macromolecules 1993, 26, 539–544. [Google Scholar] [CrossRef]
- Cook, A.G.; Inkster, R.T.; Martinez-Felipe, A.; Ribes-Greus, A.; Hamley, I.W.; Imrie, C.T. Synthesis and phase behaviour of a homologous series of polymethacrylate-based side-chain liquid crystal polymers. Eur. Polym. J. 2012, 48, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Pebalk, D.; Barmatov, E.; Shibayev, V. Liquid crystalline ionomers as a new class of mesomorphous polymeric systems. Usp. Khim. 2005, 74, 610–633. [Google Scholar] [CrossRef]
- Kumar, G.; Neckers, D. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Concellon, A.; Blasco, E.; Martinez-Felipe, A.; Carlos Martinez, J.; Sics, I.; Ezquerra, T.A.; Nogales, A.; Pinol, M.; Oriol, L. Light-responsive self-assembled materials by supramolecular post-functionalization via hydrogen bonding of amphiphilic block copolymers. Macromolecules 2016, 49, 7825–7836. [Google Scholar] [CrossRef]
- Merino, E.; Ribagorda, M. Control over molecular motion using the cis-trans photoisomerization of the azo group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. [Google Scholar] [CrossRef] [Green Version]
- Kamogawa, H. Redox behavior in photochromic polymers of thiazine series. J. Appl. Polym. Sci. 1969, 13, 1883. [Google Scholar] [CrossRef]
- Kamogawa, H.; Kato, M.; Sugiyama, H. Syntheses and properties of photochromic polymers of azobenzene and thiazine series. J. Polym. Sci. Part A Polym. Chem. 1968, 6, 2967. [Google Scholar] [CrossRef]
- Chaganava, I.; Kobulashvili, I.; Alauddin, M.S.; Aripin, N.F.K.; Martinez-Felipe, A. Light-inducing birefringence of organic photoanisotropic materials integrated via covalent bonds. In Organic Photonic Materials and Devices XXI; Kajzar, F., Kaino, T., Tabor, C.E., Eds.; SPIE: Bellingham, WA, USA, 2019; Volume 10915. [Google Scholar]
- Chaganava, I.; Kilosanidze, B.; Kakauridze, G.; Oriol, L.; Pinol, M.; Martinez-Felipe, A. Induction of the vector polyphotochromism in side-chain azopolymers. J. Photochem. Photobiol. A Chem. 2018, 354, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R. On the static permittivity of dipolar and conductive media—An educational-approach. J. Non Cryst. Solids 1991, 131, 1136–1139. [Google Scholar] [CrossRef]
- Liang, T.; van Kuringen, H.P.C.; Mulder, D.J.; Tan, S.; Wu, Y.; Borneman, Z.; Nijmeijer, K.; Schenning, A.P.H.J. Anisotropic dye adsorption and anhydrous proton conductivity in smectic liquid crystal networks: The role of cross-link density, order, and orientation. ACS Appl. Mater. Interfaces 2017, 9, 35218–35225. [Google Scholar] [CrossRef]
- Yang, X.; Tan, S.; Liang, T.; Wei, B.; Wu, Y. A unidomain membrane prepared from liquid-crystalline poly(pyridinium 4-styrene sulfonate) for anhydrous proton conduction. J. Membr. Sci. 2017, 523, 355–360. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ichikawa, T.; Kato, T.; Ohno, H. Development of glassy bicontinuous cubic liquid crystals for solid proton-conductive materials. Adv. Mater. 2017, 29, 1604429. [Google Scholar] [CrossRef]
- Concellon, A.; Liang, T.; Schenning, A.P.H.J.; Luis Serrano, J.; Romero, P.; Marcos, M. Proton-conductive materials formed by coumarin photocrosslinked ionic liquid crystal dendrimers. J. Mater. Chem. C 2018, 6, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Koduru, H.K.; Marinov, Y.G.; Hadjichristov, G.B.; Scaramuzza, N. Characterization of polymer/liquid crystal composite based electrolyte membranes for sodium ion battery applications. Solid State Ion. 2019, 335, 86–96. [Google Scholar] [CrossRef]
- Nederstedt, H.; Jannasch, P. Single-ion conducting polymer electrolytes with alternating ionic mesogen-like moieties interconnected by poly(ethylene oxide) segments. Polymer 2019, 177, 231–240. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Santonja-Blasco, L.; Badia, J.D.; Imrie, C.T.; Ribes-Greus, A. Characterization of functionalized side-chain liquid crystal methacrylates containing nonmesogenic units by dielectric spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8722–8731. [Google Scholar] [CrossRef]
- Colomer, F.; Duenas, J.; Ribelles, J.; Barralesrienda, J.; Deojeda, J. Side-chain liquid-crystalline poly(n-maleimides).5. dielectric-relaxation behavior of liquid-crystalline side-chain and amorphous poly(n-maleimides)—A comparative structural study. Macromolecules 1993, 26, 155–166. [Google Scholar] [CrossRef]
- Nikonorova, N.; Borisova, T.; Barmatov, E.; Pissis, P.; Diaz-Calleja, R. Dielectric relaxation and thermally stimulated discharge currents in liquid-crystalline side-chain polymethacrylates with phenylbenzoate mesogens having tail groups of different length. Macromolecules 2003, 36, 5784–5791. [Google Scholar] [CrossRef]
- Ribesgreus, A.; Gomezribelles, J.; Diazcalleja, R. Dielectric and mechanical dynamical studies on poly(cyclohexyl methacrylate). Polymer 1985, 26, 1849–1854. [Google Scholar] [CrossRef]
- Ribesgreus, A.; Soria, V.; Figueruelo, J.; Diazcalleja, R. Study of the molecular—Origin of the mechanical and dielectric-beta relaxation of methyl-methacrylate isopropenyl methyl ketone copolymers. Makromol. Chem. Macromol. Symp. 1988, 20, 409–416. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Farquharson, E.; Hashim, R.; Velayutham, T.S.; Aripin, N.F.K. Glycolipids from natural sources: Dry liquid crystal properties, hydrogen bonding and molecular mobility of palm kernel oil mannosides. Liq. Cryst. 2020. [Google Scholar] [CrossRef]
- Zentel, R.; Strobl, G.; Ringsdorf, H. Dielectric—Relaxation of liquid-crystalline polyacrylates and polymethacrylates. Macromolecules 1985, 18, 960–965. [Google Scholar] [CrossRef]
- Schonhals, A.; Wolff, D.; Springer, J. Influence of the mesophase structure on the beta-relaxation in comb-like polymethacrylates. Macromolecules 1995, 28, 6254–6257. [Google Scholar] [CrossRef]
- Imrie, C.; Karasz, F.E.; Attard, G.S. The effect of molecular-weight on the thermal-properties of polystyrene-based side-chain liquid-crystalline polymers. J. Macromol. Sci. Pure Appl. Chem. 1994, 31, 1221–1232. [Google Scholar] [CrossRef]
- Craig, A.; Imrie, C. Effect of spacer length on the thermal-properties of side-chain liquid-crystal poly(methacrylate)s. J. Mater. Chem. 1994, 4, 1705–1714. [Google Scholar] [CrossRef]
- Schleeh, T.; Imrie, C.T.; Rice, D.; Karasz, F.E.; Attard, G.S. Ultrastructure studies of polystyrene-based side-chain liquid-crystalline copolymers containing charge-transfer groups. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1859–1869. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the copolymers compounds are available from the authors. |










| P# | Polymer Full Name | Mn g·mol−1 | Mw g·mol−1 | Mw/Mn | Yield (%) | MeOAzB/AMPS/MMA (% molar) |
|---|---|---|---|---|---|---|
| PA | PMeOAzB25 † | 11223 | 12920 | 1.15 | 68 | 100/0/0 |
| PS | PAMPS192 † | 39863 | 73407 | 1.84 | 72 | 0/100/0 |
| PM | PMMA136 † | 13613 | 15034 | 1.10 | 70 | 0/0/100 |
| PA-b-PS | PMeOAzB25-b-PAMPS12 | 13705 | 24176 | 1.76 | 58 | 67.6/32.4/0 |
| PA-b-PS-b-PM | PMeOAzB25-b-PAMPS12-b-PMMA23 | 16054 | 33817 | 2.10 | 52 | 41.7/20.0/38.3 |
| P(A-co-S) | P(MeOAzB0.53-co-AMPS0.47)57‡ | 19192 | 60525 | 1.83 | 71 | 53.0/47.0/0 |
| P(A-co-S)-b-PM | P(MeOAzB0.53-co-AMPS0.47)57-b-PMMA119‡ | 22901 | 57429 | 2.51 | 75 | 17.2/15.2/67.6 |
| P(A-co-S-co-M) | P(MeOAzB0.18-co-AMPS0.47-co-MMA0.35)‡ | 31088 | 89357 | 2.87 | 73 | 18.0/47.0/35.0 |
| PA-b-PM | PMeOAzB25-b-PMMA22 | 13412 | 16060 | 1.22 | 55 | 53.2/0/46.8 |
| PM-b-PS | PMMA136-b-PAMPS471 | 111356 | 124294 | 1.12 | 62 | 0/77.6/22.4 |
| Polymer | Tg °C | TLCI °C | ∆HLCI J·g−1 | ∆HLCI kJ·mol−1 | ∆SLCI/R |
|---|---|---|---|---|---|
| PA | 67.7 | 130.1 | 6.30 | 2.85 | 0.85 |
| PS | 138.0 | - | - | - | - |
| PM | 99.3 | - | - | - | - |
| PA-b-PS | 77.8 | 138.9 | 3.80 | 1.41 | 0.41 |
| PA-b-PS-b-PM | 77.6 | 138.9 | 2.31 | 0.73 | 0.21 |
| P(A-co-S) | 77.3 | 135.5 | 3.96 | 1.27 | 0.37 |
| P(A-co-S)-b-PM | 119.0 | - | - | - | - |
| P(A-co-S-co-M) | 59.0 | - | - | - | - |
| PA-b-PM | 69.6 | 131.7 | 2.40 | 0.91 | 0.27 |
| PM-b-PS | 110.0 | - | - | - | - |
| P# | di Å | ||||
|---|---|---|---|---|---|
| PA | 17.58/15.81 † | 11.73 | 5.29 | ||
| PS | 10.85 | 7.00 | * | ||
| PM | 6.56 | ||||
| PA-b-PS | 15.35 † | 10.88 | 7.69 | 4.94 * | |
| PA-b-PS-b-PM | 15.25 † | 10.15 | 6.79 | 4.57 † | |
| P(A-co-S) | 17.57 † | 11.51 | 8.64 | 5.42 † | |
| P(A-co-S)-b-PM | 6.93 † | ||||
| P(A-co-S-co-M) | 7.30 † | 4.87 † | |||
| PA-b-PM | 17.48 † | 11.90 † | 5.37 † | ||
| PM-b-PS | 6.86 † | ||||
| P# | Ea kJ mol−1 | ∆T Range °C |
|---|---|---|
| PS | 16.1 | 50/100 |
| PA-b-PS | 198.2 | 90/120 |
| PA-b-PS-b-PM | 47.9 | 70/120 |
| P(A-co-S) | 65.7 | 60/100 |
| P(A-co-S)-b-PM | 156.2 | 120/150 |
| P(A-co-S-co-M) | 22.6 | 60/120 |
| PM-b-PS | 17.4 | 60/130 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Alauddin, S.; Fadhilah Kamalul Aripin, N.; Selvi Velayutham, T.; Martinez-Felipe, A. Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity. Molecules 2020, 25, 2579. https://doi.org/10.3390/molecules25112579
Mohd Alauddin S, Fadhilah Kamalul Aripin N, Selvi Velayutham T, Martinez-Felipe A. Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity. Molecules. 2020; 25(11):2579. https://doi.org/10.3390/molecules25112579
Chicago/Turabian StyleMohd Alauddin, Sakinah, Nurul Fadhilah Kamalul Aripin, Thamil Selvi Velayutham, and Alfonso Martinez-Felipe. 2020. "Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity" Molecules 25, no. 11: 2579. https://doi.org/10.3390/molecules25112579
APA StyleMohd Alauddin, S., Fadhilah Kamalul Aripin, N., Selvi Velayutham, T., & Martinez-Felipe, A. (2020). Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity. Molecules, 25(11), 2579. https://doi.org/10.3390/molecules25112579