Antifungal and Cytotoxic Evaluation of Photochemically Synthesized Heparin-Coated Gold and Silver Nanoparticles
Abstract
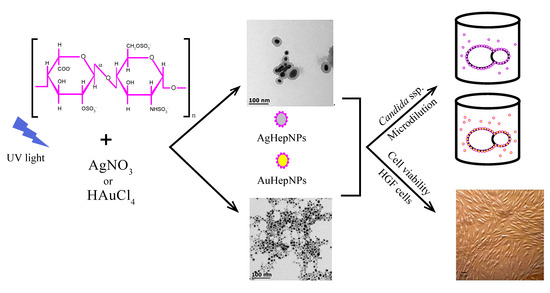
:1. Introduction
2. Results
2.1. UV–Vis Spectroscopy
2.2. Transmission Electron Microscopy
2.3. Raman Spectroscopy
2.4. Antifungal Susceptibility Test
2.5. MTT Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nanoparticle Synthesis
4.2.1. Stock Solutions Preparation
4.2.2. Synthesis Procedure
4.3. Nanoparticle Characterization
4.4. Antifungal Susceptibility Test
4.5. MTT Assay
- Non-cytotoxic (>90% cell viability);
- Slightly cytotoxic (60–90% cell viability);
- Moderately cytotoxic (30–59% cell viability);
- Severely cytotoxic (≤30% cell viability).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yang, P.; Zheng, J.; Xu, Y.; Zhang, Q.; Jiang, L. Colloidal Synthesis and Applications of Plasmonic Metal Nanoparticles. Adv. Mater. 2016, 28, 10508–10517. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Sankar, R.; Rahman, P.K.; Varunkumar, K.; Anusha, C.; Kalaiarasi, A.; Shivashangari, K.S.; Ravikumar, V. Facile synthesis of Curcuma longa tuber powder engineered metal nanoparticles for bioimaging applications. J. Mol. Struct. 2017, 1129, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Multifunctional Inorganic Nanoparticles: Recent Progress in Thermal Therapy and Imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef]
- Paramasivam, G.; Kayambu, N.; Rabel, A.M.; Sundramoorthy, A.K.; Sundaramurthy, A. Anisotropic noble metal nanoparticles: Synthesis, surface functionalization and applications in biosensing, bioimaging, drug delivery and theranostics. Acta Biomater. 2017, 49, 45–65. [Google Scholar] [CrossRef]
- Zazo, H.; Millán, C.G.; Colino, C.I.; Lanao, J.M. Chapter 15—Applications of Metallic Nanoparticles in Antimicrobial Therapy; Grumezescu, A.M.B.T.-A.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 411–444. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Henam, S.D.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A.H. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- Schneider, T.; Westermann, M.; Glei, M. In vitro uptake and toxicity studies of metal nanoparticles and metal oxide nanoparticles in human HT29 cells. Arch. Toxicol. 2017, 91, 3517–3527. [Google Scholar] [CrossRef]
- Pandey, N.; Dhiman, S.; Srivastava, T.; Majumder, S. Transition metal oxide nanoparticles are effective in inhibiting lung cancer cell survival in the hypoxic tumor microenvironment. Chem. Biol. Interact. 2016, 254, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B Biol. 2016, 164, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Schmid, G. Synthesis of Metal Nanoparticles. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Page, C. Heparin and Related Drugs: Beyond Anticoagulant Activity. ISRN Pharmacol. 2013, 2013, 910743. [Google Scholar] [CrossRef] [Green Version]
- Hirsh, J.; Dalen, J.E.; Deykin, D.; Toller, L. Heparin: Mechanism of Action, Pharmacokinetics, Dosing Considerations, Monitoring, Efficacy, and Safety. Chest 1992, 102, S337–S351. [Google Scholar] [CrossRef]
- Ludwig, R. Therapeutic Use of Heparin beyond Anticoagulation. Curr. Drug Discov. Technol. 2009, 6, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.A.; Gavalda, J.; Fortea, J.; Lopez, P.; Martin, M.T.; Gomis, X.; Pahissa, A. Lack of antimicrobial activity of sodium heparin for treating experimental catheter-related infection due to Staphylococcus aureus using the antibiotic-lock technique. Clin. Microbiol. Infect. 2001, 7, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosett, W.; Hodges, G.R. Antimicrobial activity of heparin. J. Clin. Microbiol. 1980, 11, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Miceli, M.H.; Bernardo, S.M.; Ku, T.S.N.; Walraven, C.; Lee, S.A. In Vitro Analyses of the Effects of Heparin and Parabens on Candida albicans, Biofilms and Planktonic Cells. Antimicrob. Agents Chemother. 2012, 56, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Weijmer, M.C.; Debets-Ossenkopp, Y.J.; van de Vondervoort, F.J.; ter Wee, P.M. Superior antimicrobial activity of trisodium citrate over heparin for catheter locking. Nephrol. Dial. Transplant. 2002, 17, 2189–2195. [Google Scholar] [CrossRef] [Green Version]
- Kemp, M.M.; Kumar, A.; Clement, D.; Ajayan, P.; Mousa, S.; Linhardt, R.J. Hyaluronan- and heparin-reduced silver nanoparticles with antimicrobial properties. Nanomedicine 2009, 4, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, H. Preparation and Characterization of Heparin-Stabilized Gold Nanoparticles. J. Carbohydr. Chem. 2008, 27, 309–319. [Google Scholar] [CrossRef]
- del Pilar Rodríguez-Torres, M.; Díaz-Torres, L.A.; Romero-Servin, S. Heparin assisted photochemical synthesis of gold nanoparticles and their performance as SERS substrates. Int. J. Mol. Sci. 2014, 15, 19239–19252. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Decan, M.R.; Wang, D.; Scaiano, J.C. Facile photochemical synthesis of unprotected aqueous gold nanoparticles. J. Am. Chem. Soc. 2006, 128, 15980–15981. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.C.; Eun, D.K.; Na, J.H.; Lee, S.; Kim, I.J.; Youn, I.C.; Ko, C.Y.; Kim, H.S.; Lim, D.; Choi, K.; et al. Heparin-Coated gold nanoparticles for liver-Specific CT imaging. Chem. A Eur. J. 2009, 15, 13341–13347. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, L.; Li, J. Ultrasensitive colorimetric detection of heparin based on self-assembly of gold nanoparticles on graphene oxide. Analyst 2012, 137, 3653–3658. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Mousa, S.; Park, T.J.; Ajayan, P.; Kubotera, N.; Mousa, S.A.; Linhardt, R.J. Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules 2009, 10, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Andrades, J.R.; Pérez-Gramatges, A.; Pandoli, O.; Romani, E.C.; Aucélio, R.Q.; da Silva, A.R. Spherical gold nanoparticles and gold nanorods for the determination of gentamicin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 126–134. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Spencer, J.A.; Kauffman, J.F.; Reepmeyer, J.C.; Gryniewicz, C.M.; Ye, W.; Toler, D.Y.; Buhse, L.F.; Westenberger, B.J. Screening of Heparin API by Near Infrared Reflectance and Raman Spectroscopy. J. Pharm. Sci. 2009, 98, 3540–3547. [Google Scholar] [CrossRef]
- Carlini, L.; Fasolato, C.; Postorino, P.; Fratoddi, I.; Venditti, I.; Testa, G.; Battocchio, C. Comparison between silver and gold nanoparticles stabilized with negatively charged hydrophilic thiols: SR-XPS and SERS as probes for structural differences and similarities. Colloids Surf. Physicochem. Eng. Asp. 2017, 532, 183–188. [Google Scholar] [CrossRef]
- Kumar, G.V.P.; Shruthi, S.; Vibha, B.; Reddy, B.A.A.; Kundu, T.K.; Narayana, C. Hot Spots in Ag Core−Au Shell Nanoparticles Potent for Surface-Enhanced Raman Scattering Studies of Biomolecules. J. Phys. Chem. C 2007, 111, 4388–4392. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Ribeiro, M.R.; Pontes, M.J.; Nogueira, B.V.; Guimarães, M.C. A helpful method for controlled synthesis of monodisperse gold nanoparticles through response surface modeling. Arab. J. Chem. 2020, 13, 216–226. [Google Scholar] [CrossRef]
- Sjögren, G.; Sletten, G.; Dahl, J.E. Cytotoxicity of dental alloys, metals, and ceramics assessed by Millipore filter, agar overlay, and MTT tests. J. Prosthet. Dent. 2000, 84, 229–236. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2018, 25, 10362–10370. [Google Scholar] [CrossRef]
- Abdel Rahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.-Z.M.A.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Giorgi-Coll, S.; Blunt-Foley, H.; Hutchinson, P.J.; Carpenter, K.L.H. Heparin-gold nanoparticles for enhanced microdialysis sampling. Anal. Bioanal. Chem. 2017, 409, 5031–5042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bener, M.; Şen, F.B.; Apak, R. Heparin-stabilized gold nanoparticles-based CUPRAC colorimetric sensor for antioxidant capacity measurement. Talanta 2018, 187, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Mamidyala, S.K. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2011, 84, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neihof, R.A.; Bailey, C.A. Biocidal properties of anti-icing additives for aircraft fuels. Appl. Environ. Microbiol. 1978, 35, 698–703. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Mohd Zaidi, N.F.; Valsami-Jones, E.; Kreft, J.-U. Time-resolved toxicity study reveals the dynamic interactions between uncoated silver nanoparticles and bacteria. Nanotoxicology 2017, 11, 637–646. [Google Scholar] [CrossRef]
- Green, J.V.; Orsborn, K.I.; Zhang, M.; Tan, Q.K.; Greis, K.D.; Porollo, A.; Andes, D.R.; Long Lu, J.; Hostetter, M.K. Heparin-Binding Motifs and Biofilm Formation by Candida albicans. J. Infect. Dis. 2013, 208, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, U.T.; Rao, G.V.S.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Comparative study of antifungal activity of silver and gold nanoparticles synthesized by facile chemical approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.V.S.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by trisodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, A.; Amalraj, T.; Devanathan, C.P.G.; Balasubramanian, T. Antimicrobial Activity of Sulfated Mucopolysaccharides [Heparin and Heparin-Like Glycosaminoglycans (GAGs)] from Cuttlefish Euprymna berryi Sasaki 1929. Trends. Appl. Sci. Res. 2011, 3, 97–102. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Holguín-Meráz, C.; Zaragoza-Contreras, E.A.; Martínez-Martínez, R.E.; Donohue-Cornejo, A.; Loyola-Rodríguez, J.P.; Cuevas-González, J.C.; Reyes-López, S.Y. Antimicrobial and Substantivity Properties of Silver Nanoparticles against Oral Microbiomes Clinically Isolated from Young and Young-Adult Patients. J. Nanomater. 2019, 2019, 3205971. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Heal. Part C 2015, 33, 286–327. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [Green Version]
- Andersson, E.; Rydengård, V.; Sonesson, A.; Mörgelin, M.; Björck, L.; Schmidtchen, A. Antimicrobial activities of heparin-binding peptides. Eur. J. Biochem. 2004, 271, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ganesan, S. In Vitro Cytotoxicity Assay on Gold Nanoparticles with Different Stabilizing Agents. J. Nanomater. 2012, 2012, 734398. [Google Scholar] [CrossRef] [Green Version]
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111715. [Google Scholar] [CrossRef]
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, 1677–1687. [Google Scholar] [CrossRef] [Green Version]
- Niska, K.; Knap, N.; Kędzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping Agent-Dependent Toxicity and Antimicrobial Activity of Silver Nanoparticles: An In Vitro Study. Concerns about Potential Application in Dental Practice. Int. J. Med. Sci. 2016, 13, 772–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurbuz, H.A.; Durukan, A.B.; Sevim, H.; Ergin, E.; Gurpinar, A.; Yorgancioglu, C. Heparin toxicity in cell culture: A critical link in translation of basic science to clinical practice. Blood Coagul. Fibrinolysis 2013, 24, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Seciu, A.M.; Manoiu, V.S.; Trif, M.; Moisei, M.; Nicu, A.I.; Zarnescu, O. Biosynthesis of silver nanoparticles in collagen gel improves their medical use in periodontitis treatment. Part. Sci. Technol. 2019, 37, 757–763. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar] [CrossRef] [Green Version]
- Krawinkel, J.; Torres-Mapa, M.L.; Werelius, K.; Heisterkamp, A.; Rüttermann, S.; Romanos, G.E.; Gerhardt-Szép, S. Gold nanoparticle-mediated delivery of molecules into primary human gingival fibroblasts using ns-laser pulses: A pilot study. Materials 2016, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Braz, A.K.S.; de Araujo, R.E.; Ohulchanskyy, T.Y.; Shukla, S.; Bergey, E.J.; Gomes, A.S.; Prasad, P.N. In situ gold nanoparticles formation: Contrast agent for dental optical coherence tomography. J. Biomed. Opt. 2012, 17, 066003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Diluition Antifungal Susceptibility Testing of Yeasts; Approved Standard, 3rd ed.; CLSI: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- ISO, B. 10993-5: Biological evaluation of Medical Devices-Part 5. In Tests for in Vitro Cytotoxicity, 2nd ed.; ISO: London, UK, 1999; Volume 1999. [Google Scholar]
- Vishwakarma, N.K.; Patel, V.K.; Hira, S.K.; Ramesh, K.; Srivastava, P.; Mitra, K.; Singh, S.; Chattopadhyay, D.; Maiti, P.; Misra, N.; et al. Tadpole-shaped β-cyclodextrin-tagged poly(N-vinylpyrrolidone): Synthesis, characterization and studies of its complexation with phenolphthalein and antitumor activities. RSC Adv. 2015, 5, 15547–15558. [Google Scholar] [CrossRef]
- Kong, N.; Jiang, T.; Zhou, Z.; Fu, J. Cytotoxicity of polymerized resin cements on human dental pulp cells in vitro. Dent. Mater. 2009, 25, 1371–1375. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Tested Agent | Candida parapsilosis MIC/MFC (μg/mL) | Issatchenkia orientalis MIC/MFC (μg/mL) | Candida albicans MIC/MFC (μg/mL) |
|---|---|---|---|
| Itraconazole | 0.250/0.250 | 0.50/0.50 | >16 (resistant) |
| AgNPs (non-coated) | 3.125/12.5 | 3.125/12.5 | 6.25/>25 |
| AgHep-NPs | 3.125/>25 | 6.250/>25 | 25/>25 |
| AuNPs (non-coated) | >25 | >25 | >25 |
| AuHep-NPs | >25 | >25 | >25 |
| Heparin solution | >1000/>1000 | >1000/>1000 | >1000/>1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Torres, M.d.P.; Díaz-Torres, L.A.; Millán-Chiu, B.E.; García-Contreras, R.; Hernández-Padrón, G.; Acosta-Torres, L.S. Antifungal and Cytotoxic Evaluation of Photochemically Synthesized Heparin-Coated Gold and Silver Nanoparticles. Molecules 2020, 25, 2849. https://doi.org/10.3390/molecules25122849
Rodriguez-Torres MdP, Díaz-Torres LA, Millán-Chiu BE, García-Contreras R, Hernández-Padrón G, Acosta-Torres LS. Antifungal and Cytotoxic Evaluation of Photochemically Synthesized Heparin-Coated Gold and Silver Nanoparticles. Molecules. 2020; 25(12):2849. https://doi.org/10.3390/molecules25122849
Chicago/Turabian StyleRodriguez-Torres, María del Pilar, Luis Armando Díaz-Torres, Blanca E. Millán-Chiu, René García-Contreras, Genoveva Hernández-Padrón, and Laura Susana Acosta-Torres. 2020. "Antifungal and Cytotoxic Evaluation of Photochemically Synthesized Heparin-Coated Gold and Silver Nanoparticles" Molecules 25, no. 12: 2849. https://doi.org/10.3390/molecules25122849
APA StyleRodriguez-Torres, M. d. P., Díaz-Torres, L. A., Millán-Chiu, B. E., García-Contreras, R., Hernández-Padrón, G., & Acosta-Torres, L. S. (2020). Antifungal and Cytotoxic Evaluation of Photochemically Synthesized Heparin-Coated Gold and Silver Nanoparticles. Molecules, 25(12), 2849. https://doi.org/10.3390/molecules25122849