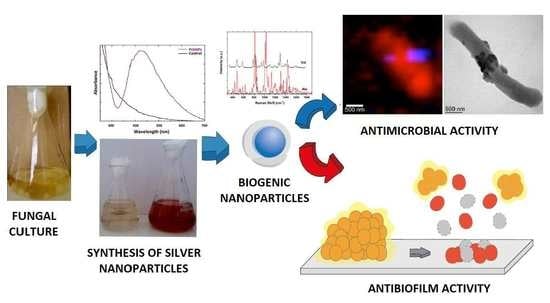
Biofilm Eradication Using Biogenic Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Biogenic Silver Nanoparticles (PchNPs)
2.2. Evaluation of the Incidence of Reaction Conditions in the Biosynthesis of PchNPs
2.3. Characterization of PchNPs
2.3.1. ζ-potential: Surface Charge of PchNPs
2.3.2. DLS and SAXS: Diameter of PchNPs
2.3.3. Confocal Raman Microscopy: Surface Functional Groups of PchNPs
2.4. Colloidal Stability Assays
2.5. Antimicrobial Activity of PchNPs against E. coli
2.5.1. Antibacterial Activity against E. coli
2.5.2. ESEM and TEM of E. coli Cells Exposed to PchNPs
2.6. Antimicrobial Properties of PchNPs Using Confocal Raman Microscopy
2.7. Antibiofilm Activiy of PchNPs
3. Materials and Methods
3.1. Synthesis of Silver Nanoparticles
3.2. Characterization of Silver Nanoparticles
3.2.1. UV-Vis Spectroscopy
3.2.2. ζ-Potential and DYNAMIC Light Scattering (DLS)
3.2.3. Small Angle X-ray Scattering (SAXS)
3.2.4. Confocal Raman Microscopy
3.2.5. Colloidal Stability Assays
3.3. Antimicrobial Activity of PchNPs
3.3.1. Antibacterial Activity against E. coli
Enviromental Scanning Electron Microscopy (ESEM)
Transmission Electron Microscopy (TEM)
3.3.2. Antimicrobial Properties of PchNPs Using Confocal Raman Microscopy
3.4. Antibiofilm Activiy of PchNPs
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. CellsNanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.N. Kava: An overview. J. Ethnopharmacol. 1992, 37, 13–45. [Google Scholar] [CrossRef]
- Alamri, S.A.M.; Hashem, M.; Nafady, N.A.; Sayed, M.A.; Alshehri, A.M.; Alshaboury, G. Controllable biogenic synthesis of intracellular Silver/Silver Chloride Nanoparticles by Meyerozyma guilliermondii KX008616. J. Microbiol. Biotechnol. 2018, 28, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javani, S.; Marín, I.; Amils, R.; Abad, J.P. Four psychrophilic bacteria from Antarctica extracellularly biosynthesize at low temperature highly stable silver nanoparticles with outstanding antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 60–69. [Google Scholar] [CrossRef]
- Khan, M.R.; Fromm, K.M.; Rizvi, T.F.; Giese, B.; Ahamad, F.; Turner, R.J.; Füeg, M.; Marsili, E. Metal Nanoparticle–Microbe Interactions: Synthesis and Antimicrobial Effects. Part. Part. Syst. Charact. 2020, 1900419. [Google Scholar] [CrossRef]
- Su, D.-L.; Li, P.-J.; Ning, M.; Li, G.-Y.; Shan, Y. Microwave assisted green synthesis of pectin based silver nanoparticles and their antibacterial and antifungal activities. Mater. Lett. 2019, 244, 35–38. [Google Scholar] [CrossRef]
- Sanguiñedo, P.; Fratila, R.M.; Estevez, M.B.; Martínez de la Fuente, J.; Grazú, V.; Alborés, S. Extracellular Biosynthesis of Silver Nanoparticles Using Fungi and Their Antibacterial Activity. Nano Biomed. Eng. 2018, 10, 156–164. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Neethu, S.; Midhun, S.J.; Radhakrishnan, E.K.; Jyothis, M. Surface functionalization of central venous catheter with mycofabricated silver nanoparticles and its antibiofilm activity on multidrug resistant Acinetobacter baumannii. Microb. Pathog. 2020, 138. [Google Scholar] [CrossRef]
- Markowska, K.; Grudniak, A.M.; Wolska, K.I. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013, 60, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bhargava, A. Biofilms and human health. Biotechnol. Lett. 2016, 38, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef]
- Luan, Y.; Liu, S.; Pihl, M.; van der Mei, H.C.; Liu, J.; Hizal, F.; Choi, C.-H.; Chen, H.; Ren, Y.; Busscher, H.J. Bacterial interactions with nanostructured surfaces. Curr. Opin. Colloid Interface Sci. 2018, 38, 170–189. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Xuan Nguyen, N.T.; Sarter, S.; Hai Nguyen, N.; Daniel, P. Detection of molecular changes induced by antibiotics in Escherichia coli using vibrational spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Quinteros, M.A.; Bonilla, J.O.; Alborés, S.V.; Villegas, L.B.; Páez, P.L. Biogenic nanoparticles: Synthesis, stability and biocompatibility mediated by proteins of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2019, 184. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. CellsNanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, P.R.; Whitmer, G.R.; Yoon, M.J.; Lombardo, M.N.; Pusztai-Carey, M.; Heidari-Torkabadi, H.; Che, T. Measuring Drug-Induced Changes in Metabolite Populations of Live Bacteria: Real Time Analysis by Raman Spectroscopy. J. Phys. Chem. B 2018, 122, 6377–6385. [Google Scholar] [CrossRef] [PubMed]
- Sood, R. Improved yield of green synthesized crystalline silver nanoparticles with potential antioxidant activity. Int. Res. J. Pharm. 2017, 8, 5. [Google Scholar] [CrossRef]
- Castillo, H.A.P.; Castellanos, L.N.M.; Chamorro, R.M.; Martínez, R.R.; Borunda, E.O. Nanoparticles as New Therapeutic Agents against Candida albicans. Candida Albicans 2018. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.S.; Umesha, S.; Prasad, K.S.; Niranjana, P. Detection of Quorum Sensing Molecules and Biofilm Formation in Ralstonia solanacearum. Curr. Microbiol. 2016, 72, 297–305. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 10th ed.; CLSI: Wayne, PA, USA, 2015; p. 35. [Google Scholar]
Sample Availability: Samples of the PchNPs are available from the authors. |










| Microorganism | Raman Shift (cm−1) | Band Assignment | Effect on Treated Cells |
|---|---|---|---|
| E. coli | 624 | Nucleic acids | Diminishes |
| 810 | Aminoacids | Diminishes | |
| 982 | Polysaccharides | No changes | |
| 1186 | Polysaccharides | Diminishes | |
| 1243 | Polysaccharides | Diminishes | |
| 1308 | Aminoacids or nucleic acids | Diminishes | |
| 1344 | Aminoacids or fatty acids | No changes | |
| 1593 | Fatty acids | Diminishes | |
| C. albicans | 670 | Nucleic acids | Diminishes |
| 788 | Aminoacids | Diminishes | |
| 885 | Aminoacids | Diminishes | |
| 999 | Polysaccharides | Diminishes | |
| 1100 | Polysaccharides | Diminishes | |
| 1319 | Aminoacids or nucleic acids | Diminishes | |
| 1596 | Fatty acids | Diminishes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. https://doi.org/10.3390/molecules25092023
Estevez MB, Raffaelli S, Mitchell SG, Faccio R, Alborés S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules. 2020; 25(9):2023. https://doi.org/10.3390/molecules25092023
Chicago/Turabian StyleEstevez, María Belén, Sofía Raffaelli, Scott G. Mitchell, Ricardo Faccio, and Silvana Alborés. 2020. "Biofilm Eradication Using Biogenic Silver Nanoparticles" Molecules 25, no. 9: 2023. https://doi.org/10.3390/molecules25092023
APA StyleEstevez, M. B., Raffaelli, S., Mitchell, S. G., Faccio, R., & Alborés, S. (2020). Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules, 25(9), 2023. https://doi.org/10.3390/molecules25092023