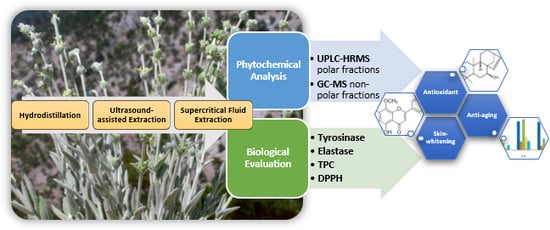
Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrodistillation and Extraction
2.2. Chemical Analysis of Different Extracts
2.2.1. GC-MS Analysis
2.2.2. UPLC-HRMS Analysis
2.3. Antioxidant Capacity
2.4. Tyrosinase and Elastase Inhibitory Activity
3. Materials and Methods
3.1. Reagents and Materials
3.2. Plant Material
3.3. Hydrodistilation (HD) Procedure
3.4. Supercritical Fluid Extraction (SFE)
3.5. Ultrasound-assisted Extraction (UAE)
3.6. Analysis of S. sipylea Extracts
3.6.1. Gas Chromatography-Mass Spectrometry (GC-MS)
3.6.2. Ultra-Performance Liquid Chromatography Coupled with High Resolution Mass Spectrometry (UPLC-HRMS)
3.7. Assessment of the Total Phenolic Content (TPC) of Extracts
3.8. DPPH• Radical Scavenging Assay
3.9. Tyrosinase Inhibition Activity
3.10. Elastase Inhibition Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1982; pp. 178–199. [Google Scholar]
- González-Burgos, E.; Carretero, M.E.; Gòmez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities-a review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef]
- Strid, A.; Tan, K. Mountain Flora of Greece; Edinburg University Press: Edinburgh, UK, 1991; pp. 84–91. [Google Scholar]
- Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Ina Aneva, I.; Evstatieva, L.N. Chemotaxonomic contribution to the Sideritis species dilemma on the Balkans. Biochem. Syst. Ecol. 2015, 61, 477–487. [Google Scholar] [CrossRef]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef]
- Romanucci, V.; Di Fabio, G.; D’Alonzo, D.; Guaragna, A.; Scapagnini, G.; Zarelli, A. Traditional uses, chemical composition and biological activities of Sideritis raeseri Boiss. & Heldr. J. Sci. Food Agric. 2017, 97, 373–383. [Google Scholar] [CrossRef]
- Villar, A.; Jiménez, M.J.; Alcaraz, M.J. The anti-inflammatory activity of the genus Sideritis: A new insight. Plantes MÉDicinales Et PhytothÉRapie 1986, 20, 31–36. [Google Scholar]
- Villar, A.; Recio, M.C.; Rìos, J.L.; Zafra-Polo, M.C. Antimicrobial activity of essential oils from Sideritis species. Pharmazie 1986, 41, 31–36. [Google Scholar]
- Aligiannis, N.; Kalpoutzakis, E.; Chinou, I.; Mitakou, S.; Gikas, E.; Tsarbopoulos, A. Composition and antimicrobial activity of the essential oils of five taxa of Sideritis from Greece. J. Agric. Food Chem. 2001, 49, 811–815. [Google Scholar] [CrossRef]
- Aboutabl, E.A.; Nassar, M.I.; Elsakhawy, F.M.; Maklad, Y.A.; Osman, A.F.; El-Khrisy, E.A. Phytochemical and pharmacological studies on Sideritis taurica Stephan ex Wild. J. Ethnopharmacol. 2002, 82, 177–184. [Google Scholar] [CrossRef]
- Basile, A.; Senatore, F.; Gargano, R.; Sorbo, S.; Del Pezzo, M.; Lavitola, A.; Ritieni, A.; Bruno, M.; Spatuzzi, D.; Rigano, D.; et al. Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 2006, 107, 240–248. [Google Scholar] [CrossRef]
- Kassi, E.; Paliogianni, A.; Dontas, N.; Aligiannis, N.; Halabalaki, M.; Papoutsi, Z.; Skaltsounis, L.A.; Moutsatsou, P. Effects of Sideritis euboea (Lamiaceae) aqueous extract on IL-6, OPG and RANKL secretion by osteoblasts. Nat. Prod. Commun. 2011, 6, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Tadić, V.; Oliva, A.; Božović, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar]
- Kessler, A.; Sahin-Nadeem, H.; Lummis, S.C.; Weigel, I.; Pischetsrieder, M.; Buettner, A.; Villmann, C. GABA (A) receptor modulation by terpenoids from Sideritis extracts. Mol. Nutr. Food Res. 2014, 58, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Sahin, S.; Eulenburg, V.; Kreis, W.; Villmann, C.; Pischetsrieder, M. Three-step test system for the identification of novel GABAA receptor modulating food plants. Plant. Foods Hum. Nutr. 2016, 71, 355–360. [Google Scholar] [CrossRef]
- Gonzàlez-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Kaurane diterpenes from Sideritis spp. exert a cytoprotective effect against oxidative injury that is associated with modulation of the Nrf2 system. Phytochemistry 2013, 93, 116–123. [Google Scholar] [CrossRef]
- Latté, K.P. Sideritis scardica Griseb.–Die Griechische Bergtee. Z Phytother. 2016, 37, 85–91. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Nair, A.G.; Alcaraz, M.J. Inhibition of sheep platelet arachidonate metabolism by flavonoids from Spanish and Indian medicinal herbs. Pharmazie 1990, 45, 206–208. [Google Scholar]
- Charami, M.T.; Lazari, D.; Karioti, A.; Skaltsa, H.; Hadjipavlou-Litina, D.; Souleles, C. Antioxidant and antiinflammatory activities of Sideritis perfoliata subsp. perfoliata (Lamiaceae). Phytother. Res. 2008, 22, 450–454. [Google Scholar] [CrossRef]
- Kostadinova, E.; Alipieva, K.; Stefova, M.; Antonova, D.; Evstatieva, L.; Stefkov, G.; Tsvetkova, I.; Naydesnki, H.; Bankona, V. Influence of cultivation on the chemical composition and antimicrobial activity of Sideritis spp. Phcog. Mag. 2008, 4, 102–106. [Google Scholar]
- Bruno, M.; Rosselli, S.; Pibiri, I.; Kilgore, N.; Lee, K.-H. Anti-HIV agents derived from the ent-kaurene diterpenoid linearol. J. Nat. Prod. 2002, 65, 1594–1597. [Google Scholar] [CrossRef]
- Phitos, D.; Constantinidis, T.; Kamari, G. The Red Data Book of Rare and Threatened Plants of Greece; Hellenic Βotanical Society: Patra, Greek, 2009. [Google Scholar]
- Gergis, N.; Argyriadou, N.; Poulos, C.P. Composition of the essential oils of Sideritis clandestina ssp. cyllenea and Sideritis sipylea. J. Sci. Food Agric. 1989, 47, 501–507. [Google Scholar] [CrossRef]
- Kilic, T.; Yildiz, Y.K.; Goren, A.C.; Tumen, G.; Topcu, G. Phytochemical analysis of some Sideritis species of Turkey. Chem. Nat. Compd. 2003, 39, 453–456. [Google Scholar] [CrossRef]
- Loğoğlu, E.; Arslan, S.; Öktemer, A.; Şakõyan, I. Biological activities of some natural compounds from Sideritis sipylea Boiss. Phytother. Res. 2006, 20, 294–297. [Google Scholar] [CrossRef]
- Nakiboglu, M.; Ozturk-Urek, R.; Ayar-Kayali, H.; Tarhan, L. Antioxidant capacities of endemic Sideritis sipylea and Origanum sipyleum from Turkey. Food Chem. 2007, 104, 630–635. [Google Scholar] [CrossRef]
- Tadić, V.; Bojović, D.; Arsić, I.; Đorđević, S.; Aksentijevic, K.; Stamenić, M.; Janković, S. Chemical and Antimicrobial Evaluation of Supercritical and Conventional Sideritis scardica Griseb., Lamiaceae Extracts. Molecules 2012, 17, 2683–2703. [Google Scholar] [CrossRef] [Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumnuy, A. β-caryophyllene and β-caryophyllene oxide-atural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Axiotis, E.; Halabalaki, M.; Skaltsounis, L.A. An Ethnobotanical Study of Medicinal Plants in the Greek Islands of North Aegean Region. Front. Pharmacol. 2018, 9, 409. [Google Scholar] [CrossRef]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Koleva, I.I.; Linssen, J.P.H.; van Beek, T.A.; Evstatieva, L.N.; Kortenska, V.; Handjieva, N. Antioxidant activity screening of extracts from Sideritis species (Labiatae) grown in Bulgaria. J. Sci. Food Agric. 2003, 83, 809–819. [Google Scholar] [CrossRef]
- Petreska Stanoeva, J.; Stefova, M. Assay of urinary excretion of polyphenols after ingestion of a cup of mountain tea (Sideritis scardica) measured by HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2013, 61, 10488–10497. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, C.G.; Kontogianni, V.G.; Linardaki, Z.I.; Iatrou, G.; Lamari, F.N.; Nerantzaki, A.A.; Gerothanassis, I.P.; Tzakos, A.G.; Margarity, M. Phytochemical composition of “mountain tea” from Sideritis clandestina subsp. clandestina and evaluation of its behavioral and oxidant/antioxidant effects on adult mice. Eur. J. Nutr. 2013, 52, 107–116. [Google Scholar] [CrossRef]
- Armata, M.; Gabrieli, A.; Termentzi, M.; Zervou, E.; Kokkalou, E. Constituents of Sideritis syriaca. ssp. syriaca (Lamiaceae) and their antioxidant activity. Food Chem. 2008, 111, 179–186. [Google Scholar] [CrossRef]
- Fattahi, M.; Cusido, R.M.; Khojasten, A.; Bonfill, M.; Palazon, J. Xanthomicrol: A comprehensive review of its chemistry, distribution, biosynthesis and pharmacological activity. Mini Rev. Med. Chem. 2014, 14, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Turkmenoglu, F.; Baysal, I.; Ciftci-Yabanoglu, S.; Yelekci, K.; Temel, H.; Pasa, S. Flavonoids from Sideritis species: Human monoamine oxidase (hMAO) inhibitory activities, molecular docking studies and crystal structure of Xanthomicrol. Molecules 2015, 20, 7454–7473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, G.; Ebrahim, S.A.; Rahbar-Roshandel, N.; Foroumad, A. Antiproliferative activity of flavonoids: Influence of the sequential methoxylation state of the flavonoid structure. Phytother. Res. 2011, 26, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human diseases. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 223S–229S. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 20, 1374. [Google Scholar] [CrossRef]
- Oliveira, L.L.; Carvalho, M.V.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Rev. Ceres 2014, 61, 764–779. [Google Scholar] [CrossRef] [Green Version]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgae species by linear regression analysis of spectrophotometric measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [Green Version]
- Karamian, R.; Ghasemlou, F. Screening of total phenol and flavonoid content, antioxidant and antibacterial activities of the methanolic extracts of three Silene species from Iran. Int. J. Agric. Crop. Sci. 2013, 5, 305–312. [Google Scholar]
- Hwang, J.H.; Lee, B.M. Inhibitory effects of plant extracts on tyrosinase, L-DOPA oxidation, and melanin synthesis. J. Toxicol. Environ. Health A 2007, 70, 393–407. [Google Scholar] [CrossRef]
- Deveci, E.; Tel-Çayan, G.; Emin Duru, M. Phenolic profile, antioxidant, anticholinesterase, and anti-tyrosinase activities of the various extracts of Ferula elaeochytris and Sideritis Strict. Int. J. Food Prop. 2018, 21, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef]
- Son, Y.O.; Lee, S.A.; Kim, S.S.; Jang, Y.S.; Chun, J.C.; Lee, J.C. Acteoside inhibits melanogenesis in B16F10 cells through ERK activation and tyrosinase down-regulation. J. Pharm. Pharm. 2011, 63, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Bonesi, M.; Pugliese, A.; Nadjafi, F.; Menichini, F.; Loizzo, M.R. Tyrosinase, acetyl- and butyryl-cholinesterase inhibitory activity of Stachys lavandulifolia Vahl (Lamiaceae) and Its major constituents. Rec. Nat. Prod. 2015, 9, 81–93. [Google Scholar]
- Thring, T.S.; Hill, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complem. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacem, R.; Meraihi, Z. Effects of essential oil extracted from Nigella sativa (L.) seeds and its main components on human neutrophil elastase activity. Yakugaku Zasshi 2006, 126, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lall, N.; Chrysargyris, A.; Lambrechts, I.; Fibrich, B.; Blom Van Staden, A.; Twilley, D.; de Canha, M.N.; Oosthuizen, C.B.; Bodiba, D.; Tzortzakis, N. Sideritis Perfoliata (Subsp. Perfoliata) Nutritive Value and Its Potential Medicinal Properties. Antioxidants 2019, 8, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; Blom van Staden, A.; Hosenally, M.; Mahommodally, M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R.; Žabka, M.; Bednář, J.; Tříska, J.; Vrchotová, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crop. Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Vrchotová, N.; Tříska, J. Effect of foliar nutrition on the essential oil yield of Thyme (Thymus vulgaris L.). Ind. Crop. Prod. 2018, 112, 762–765. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Çarıkçı, S.; Kılıç, T.; Azizoglu, A.; Topçu, G. Chemical Constituents of Two Endemic Sideritis Species from Turkey with Antioxidant Activity. Rec. Nat. Prod. 2012, 6, 101–109. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechol. Bioch. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacem, R. Phenolic compounds from medicinal plants as natural anti-elastase products for the therapy of pulmonary emphysema. J. Med. Plants Res. 2013, 7, 3499–3507. [Google Scholar] [CrossRef]
Sample Availability: Samples of Sideritis sipylea extracts are available from the authors. |





| Type of Extract | Solvent | Extraction Technique | Yield (%w/w) 1,2 |
|---|---|---|---|
| Essential oil (EO) | – | HD | 0.08 ± 0.02 d |
| sCO2 extract (SFE) | sCO2 | SFE | 1.65 ± 0.09 c |
| DCM extract (DCM) | DCM | UAE | 1.12 ± 0.12 cd |
| EtOAc extract (EtOAc) | EtOAc | UAE | 1.00 ± 0.08 cd |
| MeOH extract (ME) | Methanol | UAE | 12.90 ± 0.60 b |
| H2O/MeOH (50:50 v/v) (HA) | Water/Methanol | UAE | 14.64 ± 0.52 a |
| No. | RI 1 | Compound | % Peak Area 2 | ||
|---|---|---|---|---|---|
| DCM | EO | SFE | |||
| 1 | 965 | α-Pinene | 0.95 ± 0.13 | - | - |
| 2 | 988 | Sabinene | 0.10 ± 0.01 | - | - |
| 3 | 991 | β-Pinene | 0.37 ± 0.03 | - | - |
| 4 | 1082 | cis-Sabinene hydrate | 0.03 ± 0.00 | - | - |
| 5 | 1107 | Linalool | - | 0.03 ± 0.01 | - |
| 6 | 1123 | trans-p-Mentha-2,8-dien-1-ol | 0.05 ± 0.00 | - | - |
| 7 | 1141 | α-Campholenal | 0.02 ± 0.01 | - | - |
| 8 | 1154 | trans-Pinocarveol | 0.02 ±0.00 | 0.04 ± 0.00 | - |
| 9 | 1158 | cis-Verbenol | 0.04 ± 0.00 | 0.06 ± 0.01 | - |
| 10 | 1185 | Borneol | 0.02 ± 0.01 | 0.04 ± 0.02 | - |
| 11 | 1191 | Terpinen-4-ol | - | 0.07 ± 0.02 | - |
| 12 | 1208 | Myrtenol | 0.03 ± 0.00 | 0.11 ± 0.02 | - |
| 13 | 1289 | Bornyl acetate | 0.02 ± 0.00 | 0.03 ± 0.00 | - |
| 14 | 1333 | δ-Elemene | 0.03 ± 0.00 | 0.03 ± 0.00 | - |
| 15 | 1340 | α-Terpinyl acetate | - | 0.03 ± 0.01 | - |
| 16 | 1374 | α-Copaene | 0.01 ± 0.00 | 0.03 ± 0.01 | - |
| 17 | 1381 | (E)-β-Damascenone | - | 0.07 ± 0.03 | - |
| 18 | 1386 | β-Elemene | 0.03 ± 0.00 | 0.10 ± 0.02 | - |
| 19 | 1417 | β-Caryophyllene | 0.57 ± 0.02 | 1.32 ± 0.34 | 0.20 ± 0.06 |
| 20 | 1435 | (Z)-β-Farnesene | 0.04 ± 0.00 | 0.17 ± 0.03 | - |
| 21 | 1445 | (E)-β-Farnesene | 0.08 ± 0.00 | 0.54 ± 0.11 | - |
| 22 | 1453 | α-Humulene | 0.02 ± 0.00 | 0.08 ± 0.02 | 0.04 ± 0.01 |
| 23 | 1461 | α-Acoradiene | 0.03 ± 0.00 | 0.08 ± 0.01 | - |
| 24 | 1465 | 9-epi-(E)-Caryophyllene | 0.04 ± 0.01 | 0.11 ± 0.02 | 0.02 ± 0.01 |
| 25 | 1475 | ar-Curcumene | - | 0.19 ± 0.04 | - |
| 26 | 1477 | Germacrene D | 0.58 ± 0.01 | 0.97 ± 0.06 | 0.16 ± 0.04 |
| 27 | 1487 | α-Zingiberene | - | 0.12 ± 0.01 | - |
| 28 | 1490 | Bicyclogermacrene | 0.21 ± 0.01 | 0.11 ± 0.01 | 0.07 ± 0.03 |
| 29 | 1499 | β-Bisabolene | - | 0.11 ± 0.03 | - |
| 30 | 1500 | β-Curcumene | 0.04 ± 0.01 | 0.22 ± 0.04 | - |
| 31 | 1511 | δ-Cadinene | - | 0.06 ± 0.03 | - |
| 32 | 1531 | (E)-γ-Bisabolene | - | 0.05 ± 0.02 | - |
| 33 | 1536 | cis-Sesquisabinene hydrate | 0.07 ± 0.00 | 0.08 ± 0.01 | 0.05 ± 0.02 |
| 34 | 1575 | Spathulenol | 0.66 ± 0.04 | 3.02 ± 0.42 | 0.41 ± 0.05 |
| 35 | 1579 | Caryophyllene oxide | 0.17 ± 0.01 | 0.73 ± 0.06 | 0.14 ± 0.06 |
| 36 | 1606 | Humulene epoxide II | - | 0.07 ± 0.01 | 0.02 ± 0.01 |
| 37 | 1630 | Isospathulenol | 0.04 ± 0.00 | 0.19 ± 0.05 | - |
| 38 | 1636 | α-Acorenol | 0.02 ± 0.00 | 0.07 ± 0.02 | - |
| 39 | 1641 | β-Acorenol | 0.03 ± 0.01 | 0.02 ± 0.00 | - |
| 40 | 1645 | allo-Aromadendrene epoxide | - | 0.16 ± 0.01 | - |
| 41 | 1667 | epi-β-Bisabolol | - | 0.17 ± 0.05 | - |
| 42 | 1680 | α-Bisabolol | 0.17 ± 0.00 | 0.94 ± 0.20 | - |
| 43 | 1691 | Amorpha-4,9-dien-2-ol | 0.03 ± 0.00 | 0.12 ± 0.03 | - |
| 44 | 1705 | (2E,6Z)-Farnesal | - | 0.18 ± 0.04 | - |
| 45 | 1726 | (E)-Sesquilavandulyl acetate | 0.03 ± 0.01 | - | 0.02 ± 0.01 |
| 46 | 1825 | Cyclopentadecanolide | 0.04 ± 0.01 | 0.06 ± 0.00 | 0.07 ± 0.00 |
| 47 | 1835 | Hexahydrofarnesyl acetone | 0.26 ± 0.01 | 1.16 ± 0.00 | 0.20 ± 0.04 |
| 48 | 1843 | (Z)-Lanceol acetate | 0.68 ± 0.02 | 1.99 ± 0.03 | 0.46 ± 0.08 |
| 49 | 1905 | Isopimara-9(11),15-diene | 0.97 ± 0.05 | 2.52 ± 0.22 | 0.70 ± 0.12 |
| 50 | 1914 | Totarene | 2.37 ± 0.18 | 6.22 ± 0.34 | 2.18 ± 0.22 |
| 51 | 1923 | Beyerene | 0.56 ± 0.03 | 1.66 ± 0.22 | 0.40 ± 0.04 |
| 52 | 1949 | Geranyl-α-terpinene | 0.46 ± 0.00 | 0.62 ± 0.08 | 0.11 ± 0.02 |
| 53 | 1954 | Geranyl-p-cymene | 4.46 ± 0.16 | 17.49 ± 1.37 | 4.66 ± 0.33 |
| 54 | 1967 | (Z,Ζ)-Geranyl linalool | 5.20 ± 0.23 | 8.06 ± 0.63 | 2.76 ± 0.40 |
| 55 | 1976 | Dolabradiene | 0.15 ± 0.02 | 0.75 ± 0.15 | 0.22 ± 0.01 |
| 56 | 1981 | Sclarene | 0.30 ± 0.01 | 1.30 ± 0.12 | 0.26 ± 0.06 |
| 57 | 1987 | (E,Ζ)-Geranyl linalool | 1.56 ± 0.11 | 4.87 ± 0.16 | 1.31 ± 0.10 |
| 58 | 2003 | (Ζ,E)-Geranyl linalool | 0.47 ± 0.04 | 1.43 ± 0.08 | 0.32 ± 0.05 |
| 59 | 2015 | 13-epi-Dolabradiene | 0.28 ± 0.01 | 1.88 ± 0.10 | 0.21 ± 0.04 |
| 60 | 2028 | 13-epi-Manool oxide | - | 0.66 ± 0.08 | 0.09 ± 0.01 |
| 61 | 2033 | (E,E)-Geranyl linalool | 0.34 ± 0.01 | 1.60 ± 0.15 | 0.28 ± 0.06 |
| 62 | 2059 | Manool | 0.72 ± 0.04 | 2.17 ± 0.25 | 0.63 ± 0.12 |
| 63 | 2071 | 13-epi-Manool | 0.52 ± 0.05 | 1.67 ± 0.13 | 0.46 ± 0.08 |
| 64 | 2105 | Phytol | 0.58 ± 0.02 | 1.46 ± 0.22 | 0.53 ± 0.11 |
| 65 | 2158 | Abienol | 2.42 ± 0.13 | 1.68 ± 0.40 | 2.18 ± 0.38 |
| 66 | 2173 | Abieta-8(14),13(15)-diene | 0.72 ± 0.02 | 5.71 ± 0.88 | 0.62 ± 0.08 |
| 67 | 2185 | Sandaracopimarinal | 0.50 ± 0.01 | 1.73 ± 0.33 | 0.51 ± 0.02 |
| 68 | 2238 | Sclareol | 0.19 ± 0.02 | 0.24 ± 0.08 | 0.19 ± 0.06 |
| 69 | 2259 | 7-α-hydroxy-Manool | 0.18 ± 0.03 | 0.54 ± 0.11 | 0.21 ± 0.01 |
| 70 | 2301 | 3-α-hydroxy-Manool | 0.61 ± 0.04 | 0.76 ± 0.16 | 0.36 ± 0.05 |
| 71 | 2343 | Isopimarol | 0.29 ± 0.01 | 0.69 ± 0.18 | 0.21 ± 0.02 |
| 72 | 2580 | Sideridiol | 4.77 ± 0.66 | 0.22 ± 0.03 | 5.92 ± 0.88 |
| 73 | 2584 | n-Hexacosane | - | - | 8.87 ± 0.45 |
| 74 | 2596 | 7-Epicandicandiol | 3.53 ± 0.05 | 1.48 ± 0.17 | 3.89 ± 0.28 |
| 75 | 2639 | Siderol | 2.43 ± 0.32 | 0.54 ± 0.14 | 1.88 ± 0.30 |
| 76 | 2694 | n-Heptacosane | 3.63 ± 0.28 | 0.63 ± 0.15 | 3.91 ± 0.38 |
| 77 | 2802 | n-Octacosane | 0.97 ± 0.06 | 0.06 ± 0.02 | 1.09 ± 0.16 |
| 78 | 2929 | n-Nonacosane | 29.38 ± 1.75 | 0.95 ± 0.08 | 30.17 ± 2.03 |
| 79 | 2958 | Sidol | 5.34 ± 0.52 | - | 4.50 ± 0.35 |
| Hydrocarbon compounds | 47.35 | 44.08 | 53.89 | ||
| Oxygenated compounds | 32.08 | 39.24 | 27.60 | ||
| Monoterpene hydrocarbons | 1.42 | - | - | ||
| Oxygenated monoterpenes | 0.23 | 0.41 | - | ||
| Sesquiterpene hydrocarbons | 1.68 | 4.29 | 0.49 | ||
| Oxygenated sesquiterpenes | 2.20 | 8.96 | 1.37 | ||
| Diterpene hydrocarbons | 10.27 | 38.15 | 9.36 | ||
| Oxygenated diterpenes | 29.65 | 29.80 | 26.23 | ||
| Norisoprenoids | - | 0.07 | - | ||
| Alkanes | 33.98 | 1.64 | 44.04 | ||
| Total identified (%) | 79.43 | 83.32 | 81.49 | ||
| No. | Compound | tR (min) | [M − H]− m/z | Error | Suggested | HRMS2 [M − H]− | S. sipylea | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | Formula | ME | HA | EtOAc | ||||||
| 1 | 6-O-Caffeoyl-glucose | 0.84 | 341.1095 | 1.57 | C12H21O11 | 179 * | + | + | - | - |
| 2 | Quinic acid | 0.88 | 191.0568 | 3.71 | C7 H11 O6 | 173, 127, 85 | + | + | - | - |
| 3 | Melittoside | 2.37 | 523.1672 | 0.64 | C21H31O15 | 478, 457, 287, 197 | + | + | - | [31] |
| 4 | Melittoside derivative | 2.37 | 569.1726 | 0.51 | C22H33O17 | 523, 179 | + | + | + | - |
| 5 | Unknown | 3.57 | 375.1297 | 0.16 | C16H23O10 | 213, 169, 151 | + | + | - | - |
| 6 | Chlorogenic acid | 4.69 | 353.0880 | 0.50 | C16H17O9 | 191, 179 | + | + | - | [32] |
| 7 | Unknown | 5.36 | 435.1512 | 0.81 | C18H27O12 | 389, 287, 197 | + | + | + | - |
| 8 | Feruloylquinic acid | 6.03 | 367.1036 | 0.50 | C17H19O9 | 287, 191 | + | + | - | [4] |
| 9 | Echinacoside | 6.46 | 785.2509 | −0.06 | C35H45O20 | 623, 461 | + | + | - | [4] |
| 10 | Forsythoside B | 6.58 | 755.2408 | 0.58 | C34H43O19 | 623, 593, 461 | + | + | - | [32] |
| 11 | Verbascoside | 6.78 | 623.1992 | 1.63 | C29H35O15 | 477, 461, 315 | + | + | + | [4] |
| 12 | Samioside | 6.91 | 755.2408 | 0.58 | C34H43O19 | 623, 593, 461 | + | + | - | [4] |
| 13 | Apigenin 7-O-allosyl(1→2)glucoside | 6.97 | 593.1518 | 1.01 | C27H29O15 | 431, 269 | + | + | - | [33] |
| 14 | Isoscutellarein 7-O-allosyl(1→2)glucoside | 7.11 | 609.1471 | 1.61 | C27H29O16 | 447, 429, 285 | + | + | - | [4] |
| 15 | Isoverbascoside | 7.18 | 623.1989 | 1.13 | C29H35O15 | 461, 315 | + | + | + | [4] |
| 16 | Allysonoside | 7.39 | 769.2563 | 0.31 | C35H45O19 | 637, 593, 575, 461 | + | + | - | [4] |
| 17 | Hypolaetin 7-O-[6′’’-O-acetyl]-allosyl(1→2)glucoside | 7.41 | 667.1517 | 0.12 | C29H31O18 | 625, 463, 301 | + | + | - | [32] |
| 18 | Leucoseptoside A | 7.66 | 637.2142 | 0.69 | C30H37O15 | 491, 461, 443 | + | + | - | [4] |
| 19 | Apigenin 7-O-glucoside | 7.76 | 431.0990 | 1.53 | C21H19O10 | 269 | + | + | - | [4] |
| 20 | Apigenin 7-O-[6′’-O-acetyl]-allosyl(1→2)glucoside | 8.00 | 635.1625 | 1.14 | C29H31O16 | 593, 515, 269 | + | + | - | [33] |
| 21 | Luteolin 7-O-allosyl-(1→2)-[6′’-O-acetyl]-glucoside | 8.12 | 651.1570 | 0.47 | C29H31O17 | 591, 429, 285 | + | + | + | [32] |
| 22 | 3′-O-Methylhypolaetin 7-O-[6′’’-O-acetyl]-allosyl(1→2)glucoside | 8.41 | 681.1679 | 0.90 | C30H33O18 | 639, 621, 459, 315 | + | + | + | [4] |
| 23 | 4′-O-Methylisoscutellarein 7-O-allosyl(1→2)glucoside | 8.79 | 623.1625 | 1.26 | C28H31O16 | 461, 443, 299, 284 | + | + | + | [4] |
| 24 | Martynoside | 9.33 | 651.2297 | 0.46 | C31H39O15 | 505, 475, 457 | + | + | + | [5,33] |
| 25 | 4′-O-Methylisoscutellarein 7-O-allosyl-(1→2)-[6′’-O-acetyl]-glucoside | 9.96 | 665.1728 | 0.70 | C30H33O17 | 623, 461, 299 | + | + | + | [4,32] |
| 26 | Isoscutellarein 7-O-[6′’’-O-acetyl]-allosyl-(1→2)-[6′’-O-acetyl]-glucoside | 9.98 | 693.1644 | −4.14 | C31H33O18 | 651, 633, 471, 285 | + | - | - | [32] |
| 27 | Apigenin 7-(6′’-p-coumaroylglucoside) | 10.28 | 577.1358 | 1.02 | C30H25O12 | 431, 413, 307, 269 | + | + | - | [32] |
| 28 | Apigenin 7-(4′’-p-coumaroylglucoside) | 11.11 | 577.1356 | 0.82 | C30H25O12 | 431, 413, 307, 269 | + | + | + | [4,32] |
| 29 | 4′-O-Methylisoscutellarein 7-O-[6′’’-O-acetyl]-allosyl-(1→2)-[6′’-O-acetyl]-glucoside | 11.78 | 707.1829 | 0.05 | C32H35O18 | 665, 647, 299, 284 | + | + | + | [4] |
| 30 | Sideritoflavone | 12.04 | 359.0776 | 0.86 | C18H15O8 | 344 | - | - | + | [5] |
| 31 | Luteolin derivative | 12.54 | 313.0722 | 1.40 | C17H13O6 | 298 | + | - | + | - |
| 32 | Xanthomicrol | 13.10 | 343.0826 | 0.74 | C18H15O7 | 328, 313 | - | - | + | [5,20] |
| 33 | Unknown | 13.56 | 723.1722 | 0.35 | C39H31O14 | 577, 559, 453, 269 | + | + | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands. Molecules 2020, 25, 2022. https://doi.org/10.3390/molecules25092022
Axiotis E, Petrakis EA, Halabalaki M, Mitakou S. Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands. Molecules. 2020; 25(9):2022. https://doi.org/10.3390/molecules25092022
Chicago/Turabian StyleAxiotis, Evangelos, Eleftherios A. Petrakis, Maria Halabalaki, and Sofia Mitakou. 2020. "Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands" Molecules 25, no. 9: 2022. https://doi.org/10.3390/molecules25092022
APA StyleAxiotis, E., Petrakis, E. A., Halabalaki, M., & Mitakou, S. (2020). Phytochemical Profile and Biological Activity of Endemic Sideritis sipylea Boiss. in North Aegean Greek Islands. Molecules, 25(9), 2022. https://doi.org/10.3390/molecules25092022