10-Gingerol Targets Lipid Rafts Associated PI3K/Akt Signaling in Radio-Resistant Triple Negative Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. 10-Gingerol Suppresses the Proliferation, Migration, and Invasion of MDA-MB-231/IR Cells
2.2. 10-Gingerol Induces Apoptosis and Targets PI3K/Akt Signaling in MDA-MB-231/IR Cells
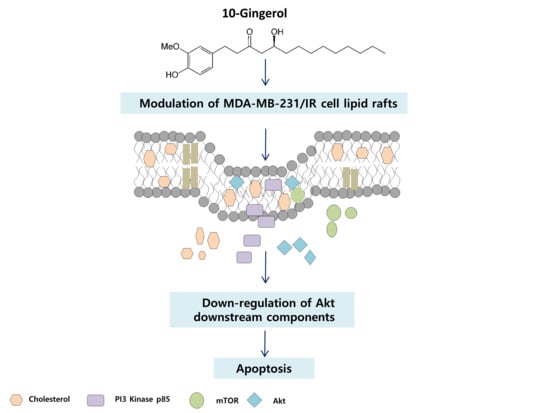
2.3. Lipid Raft Modulation by 10-Gingerol Results in Displacement of Raft-Located PI3K/Akt Signaling Components
2.4. 10-Gingerol Affects Rafts-Resident Akt Signaling and Akt Downstream Targets
3. Materials and Methods
3.1. Cell Lines, Chemicals, Reagents, Antibodies, and Kits
3.2. Cell Culture
3.3. Cell Proliferation Assay
3.4. Colony Formation Assay
3.5. Wound Healing Assay
3.6. Trans-Well Cell Invasion Assay
3.7. Western Blot Experiments
3.8. Isolation of Lipid Rafts
3.9. Immunofluorescence and Imaging
3.10. Measurement of Cholesterol Levels
3.11. Prediction of Lipophilicity of 10-gingerol
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ediriweera, M.K.; Cho, S. Targeting miRNAs by histone deacetylase inhibitors (HDACi): Rationalizing epigenetics-based therapies for breast cancer. Pharmacol. Ther. 2019, 206, 107437. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Boil. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Souchek, J.J.; Baine, M.J.; Lin, C.; Rachagani, S.; Gupta, S.; Kaur, S.; Lester, K.; Zheng, D.; Chen, S.; Smith, L.; et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br. J. Cancer 2014, 111, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Babel, L.; Kruse, L.; Bump, S.; Langhans, M.; Meckel, T. Lipid-rafts remain stable even after ionizing radiation induced disintegration of β1 integrin containing focal adhesions. BMC Res. Notes 2017, 10, 697. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Zhang, H.; Tan, Y.; Sun, C.; Liang, Y.; Yu, J.; Zou, H. Aggregation of lipid rafts activates c-met and c-Src in non-small cell lung cancer cells. BMC Cancer 2018, 18, 611. [Google Scholar] [CrossRef] [Green Version]
- Bionda, C.; Hadchity, E.; Alphonse, G.; Chapet, O.; Rousson, R.; Rodriguez-Lafrasse, C.; Ardail, D. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free. Radic. Boil. Med. 2007, 43, 681–694. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.-K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Phenethyl Isothiocyanate Suppresses Stemness in the Chemo-and Radio-Resistant Triple-Negative Breast Cancer Cell Line MDA-MB-231/IR Via Downregulation of Metadherin. Cancers 2020, 12, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Boil. 2019, 59, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Fuzer, A.M.; Martin, A.C.; Becceneri, A.B.; Da Silva, J.A.; Vieira, P.C.; Cominetti, M.R. [10]-Gingerol Affects Multiple Metastatic Processes and Induces Apoptosis in MDAMB- 231 Breast Tumor Cells. Anti-Cancer Agents Med. Chem. 2019, 19, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Thakur, K.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. 10-Gingerol, a Phytochemical Derivative from “Tongling White Ginger”, Inhibits Cervical Cancer: Insights into the Molecular Mechanism and Inhibitory Targets. J. Agric. Food Chem. 2017, 65, 2089–2099. [Google Scholar] [CrossRef]
- Rasmussen, A.; Murphy, K.; Hoskin, D.W. 10-Gingerol Inhibits Ovarian Cancer Cell Growth by Inducing G2Arrest. Adv. Pharm. Bull. 2019, 9, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zha, L.; Luo, L.; Chen, X.; Zhang, Q.; Gao, C.; Zhuang, X.; Yuan, S.; Qiao, T. [6]-Gingerol enhances the cisplatin sensitivity of gastric cancer cells through inhibition of proliferation and invasion via PI3K/AKT signaling pathway. Phytotherapy Res. 2019, 33, 1353–1362. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic Lipids, the Natural Non-isoprenoid Phenolic Amphiphiles and Their Biological Activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Stasiuk, M.; Kozubek, A. Membrane perturbing properties of natural phenolic and resorcinolic lipids. FEBS Lett. 2008, 582, 3607–3613. [Google Scholar] [CrossRef] [Green Version]
- Kozubek, A.; Nietubyc, M.; Sikorski, A.F. Modulation of the Activities of Membrane Enzymes by Cereal Grain Resorcinolic Lipids. Z. Für Nat. C 1992, 47, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Limagne, E.; Jeanningros, S.; Jacquel, A.; Lizard, G.; Athias, A.; Gambert, P.; Hichami, A.; Latruffe, N.; Solary, E.; et al. Endocytosis of Resveratrol via Lipid Rafts and Activation of Downstream Signaling Pathways in Cancer Cells. Cancer Prev. Res. 2011, 4, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pani, B.; Ong, H.L.; Liu, X.; Rauser, K.; Ambudkar, I.S.; Singh, B.B. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE). J. Boil. Chem. 2008, 283, 17333–17340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alawin, O.A.; Ahmed, R.A.; Dronamraju, V.; Briski, K.P.; Sylvester, P.W. γ-Tocotrienol-induced disruption of lipid rafts in human breast cancer cells is associated with a reduction in exosome heregulin content. J. Nutr. Biochem. 2017, 48, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Chen, C.Y.; Chen, Y.P.; Huang, Y.-B.; Lin, M.W.; Wu, D.-C.; Huang, H.T.; Liu, M.Y.; Chang, H.W.; Kao, Y.C.; et al. Betulinic acid enhances TGF-β signaling by altering TGF-β receptors partitioning between lipid-raft/caveolae and non-caveolae membrane microdomains in mink lung epithelial cells. J. Biomed. Sci. 2016, 23, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, M.; Battista, N.; Fezza, F.; Finazzi-Agro, A.; Maccarrone, M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells implications for anandamide-induced apoptosis. J. Biol. Chem. 2005, 280, 12212–12220. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, H.; Fujimura, Y.; Yamada, K. Tea polyphenol epigallocatechin-3-gallate associates with plasma membrane lipid rafts: Lipid rafts mediate anti-allergic action of the catechin. BioFactors 2004, 21, 383–385. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2012, 88, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, M.; Kuroda, K.; Ramamoorthy, A.; Yasuhara, K. Modulation of raft domains in a lipid bilayer by boundary-active curcumin. Chem. Commun. 2014, 50, 3427–3430. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Guo, X.; Wu, L.; Yang, M.; Li, Z.; Gao, Y.; Liu, S.; Zhou, G.; Zhao, J. Lipid rafts promote liver cancer cell proliferation and migration by up-regulation of TLR7 expression. Oncotarget 2016, 7, 63856–63869. [Google Scholar] [CrossRef] [Green Version]
- Wessel, D.; Flügge, U. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Luo, J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009, 273, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lam, J.B.; Lam, K.; Liu, J.; Lam, M.C.; Hoo, R.L.-C.; Wu, D.; Cooper, G.J.S.; Xu, A. Adiponectin Modulates the Glycogen Synthase Kinase-3/-Catenin Signaling Pathway and Attenuates Mammary Tumorigenesis of MDA-MB-231 Cells in Nude Mice. Cancer Res. 2006, 66, 11462–11470. [Google Scholar] [CrossRef] [Green Version]
- Eren, D.; Yerer, M.B. Revealing the effect of 6-gingerol, 6-shogaol and curcumin on mPGES-1, GSK-3β and β-catenin pathway in A549 cell line. Chem. Interact. 2016, 258, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Lee, M.-H.; Oi, N.; Kim, S.-H.; Bae, K.B.; Huang, Z.; Kim, D.J.; Reddy, K.; Lee, S.-Y.; Park, S.J. [6]-Shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2. Carcinogenesis 2014, 35, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankarshanan, M.; Ma, Z.; Iype, T.; Lorenz, U. Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 1. J. Immunol. 2007, 179, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ediriweera, M.K.; Moon, J.Y.; Nguyen, Y.T.-K.; Cho, S.K. 10-Gingerol Targets Lipid Rafts Associated PI3K/Akt Signaling in Radio-Resistant Triple Negative Breast Cancer Cells. Molecules 2020, 25, 3164. https://doi.org/10.3390/molecules25143164
Ediriweera MK, Moon JY, Nguyen YT-K, Cho SK. 10-Gingerol Targets Lipid Rafts Associated PI3K/Akt Signaling in Radio-Resistant Triple Negative Breast Cancer Cells. Molecules. 2020; 25(14):3164. https://doi.org/10.3390/molecules25143164
Chicago/Turabian StyleEdiriweera, Meran Keshawa, Jeong Yong Moon, Yen Thi-Kim Nguyen, and Somi Kim Cho. 2020. "10-Gingerol Targets Lipid Rafts Associated PI3K/Akt Signaling in Radio-Resistant Triple Negative Breast Cancer Cells" Molecules 25, no. 14: 3164. https://doi.org/10.3390/molecules25143164
APA StyleEdiriweera, M. K., Moon, J. Y., Nguyen, Y. T. -K., & Cho, S. K. (2020). 10-Gingerol Targets Lipid Rafts Associated PI3K/Akt Signaling in Radio-Resistant Triple Negative Breast Cancer Cells. Molecules, 25(14), 3164. https://doi.org/10.3390/molecules25143164