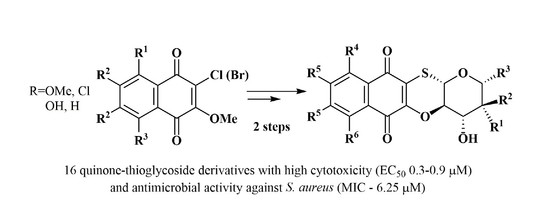
3.2.1. General Procedure for Synthesis of Acetylated Thioglycosides 13a–d–16a–d
2-Chloro(bromo)-3-methoxyquinone, 5, 6, 11, and 12 (0.50 mM), and per-O-acetylated 1-mercaptosugar derivatives 2a–d (0.55 mM) were dissolved in acetone (30 mL) and 76 mg (0.55 mM) of dry finely powdered K2CO3 was added. The resulting mixture was stirred for 2 h at room temperature until the consumption of thioglicose and conversion of starting quinone. Precipitate of inorganic salt was filtered, the filtrate was dried in a vacuum, and the residue was subjected to preparative TLC (system B for 13a–d and system A for others). The main fraction was washed off from silica gel with acetone, dried, and recrystallized from MeOH to give pure thioglycoside 13a–d–16a–d.
2-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl-1-thio)-3,5,8-trimethoxy-1,4-naphthoquinone (13a); yield 276 mg (90%), orange solid, Rf 0.37 (B), m.p. 162–165 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.97 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 2.01 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 3.70 (m, 1H, H-5′), 3.93 (s, 3H, -OCH3), 3.94 (s, 3H, -OCH3), 3.99 (dd, 1H, J = 12.4, 1.9 Hz, H-6′a), 4.14 (s, 3H, -OCH3), 4.20 (dd, 1H, J = 12.4, 4.5, Hz, H-6′b), 5.10 (t, 1H, J = 9.6 Hz, H-2′), 5.12 (t, 1H, J = 9.7 Hz, H-4′), 5.26 (t, 1H, J = 9.2 Hz, H-3′), 5.61 (d, 1H, J = 10.1 Hz, H-1′), 7.23 (d, 1H, J = 9.5 Hz, Ar-H), 7.27 (d, 1H, J = 9.5 Hz, Ar-H). 13C-NMR(125 MHz, CDCl3), δ: 20.6 (3 × COCH3), 20.7 (COCH3), 56.9 (-OCH3), 57.1 (-OCH3), 61.1 (-OCH3), 61.8 (C-6′), 68.2 (C-4′), 71.2 (C-2′), 74.1 (C-3′), 75.7 (C-5′), 80.7 (C-1′), 119.4, 120.5, 120.7, 122.2, 126.9 (C-2), 153.0, 153.7, 158.3 (C-3), 169.3 (COCH3), 169.4 (COCH3), 170.2 (COCH3), 170.6 (COCH3), 178.0, 181.4. IR (CHCl3): 3050, 2943, 2842, 1755, 1663, 1597, 1571, 1479, 1463, 1435, 1413, 1368, 1334, 1270, 1243, 1210, 1193 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C27H29O14S 609.1284, found 609.1281.
2-(2,3,4,6-Tetra-O-acetyl-β-d-galactopyranosyl-1-thio)-3,5,8-trimethoxy-1,4-naphthoquinone (13b); yield 253 mg (82.5%), orange solid, Rf 0.37 (B), m.p. 99–101 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.94 (s, 3H, COCH3), 1.98 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 2.13 (s, 3H, COCH3), 3.91 (m, 1H, H-5′), 3.93 (s, 3H, -OCH3), 3.95 (s, 3H, -OCH3), 4.02 (m, 2H, H-6′a, H-6′b), 4.14 (s, 3H, -OCH3), 5.10 (dd, 1H, J = 9.9, 3.5 Hz, H-3′), 5.31 (t, 1H, J = 10.0 Hz, H-2′), 5.42 (m, 1H, H-4′), 5.59 (d, 1H, J = 10.2 Hz, H-1′), 7.23 (d, 1H, J = 9.5 Hz, Ar-H), 7.27 (d, 1H, J = 9.5 Hz, Ar-H). 13C-NMR(125 MHz, CDCl3), δ: 20.5 (COCH3), 20.6 (2 × COCH3), 20.8 (COCH3), 56.9 (-OCH3), 57.0 (-OCH3), 61.0 (C-6′), 61.1 (-OCH3), 67.2 (C-4′), 68.4 (C-2′), 72.0 (C-3′), 74.3 (C-5′), 81.5 (C-1′), 119.4, 120.5, 120.6, 122.1, 126.9 (C-2), 153.1, 153.7, 158.5 (C-3), 169.6 (COCH3), 170.0 (2 × COCH3), 170.2 (COCH3), 178.1, 181.3. IR (CHCl3): 3054, 3006, 2941, 2842, 1750, 1663, 1597, 1571, 1479, 1463, 1435, 1413, 1372, 1334, 1270, 1251, 1185, 1155, 1085, 1060, 1022 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C27H29O14S 609.1284, found 609.1283.
2-(2,3,4-Tri-O-acetyl-β-d-xylopyranosyl-1-thio)-3,5,8-trimethoxy-1,4-naphthoquinone (13c); yield 230 mg (85%), orange solid, Rf 0.39 (B), m.p. 197–199 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.04 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 3.39 (dd, 1H, J = 11.9, 8.3 Hz, H-5′a), 3.93 (s, 3H, -OCH3), 3.95 (s, 3H, -OCH3), 4.15 (s, 3H, -OCH3), 4.21 (dd, 1H, J = 11.9, 4.7 Hz, H-5′b), 4.94 (m, 1H, H-4′), 5.04 (t, 1H, J = 8.0 Hz, H-2′), 5.20 (t, 1H, J = 8.0 Hz, H-3′), 5.60 (d, 1H, J = 8.0 Hz, H-1′), 7.24 (d, 1H, J = 9.5 Hz, Ar-H), 7.27 (d, 1H, J = 9.5 Hz, Ar-H). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (3 × COCH3), 56.9 (-OCH3), 57.1 (-OCH3), 61.2 (-OCH3), 64.9 (C-5′), 68.6 (C-4′), 70.6 (C-2′), 71.7 (C-3′), 81.6 (C-1′), 119.4, 120.6, 120.8, 122.0, 126.6 (C-2), 153.3, 153.8, 159.4 (C-3), 169.4 (COCH3), 169.7 (COCH3), 169.8 (COCH3), 178.3, 181.3. IR (CDCl3): 3054, 3018, 3006, 2942, 2842, 1752, 1663, 1597, 1571, 1478, 1463, 1435, 1413, 1371, 1334, 1272, 1248, 1210, 1185, 1062, 1024 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C24H25O12S 537.1072, found 537.1073.
2-(2,3,4-Tri-O-acetyl-α-l-arabinopyranosyl-1-thio)-3,5,8-trimethoxy-1,4-naphthoquinone (13d); yield 236 mg (87.2%), dark orange solid, Rf 0.39 (B), m.p. 92–94 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.07 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 2.11 (s, 3H, COCH3), 3.64 (dd, 1H, J = 12.6, 2.3 Hz, H-5′a), 3.92 (s, 3H, -OCH3), 3.94 (s, 3H, -OCH3), 4.08 (dd, 1H, J = 12.6, 4.3 Hz, H-5′b), 4.15 (s, 3H, -OCH3), 5.14 (dd, 1H, J = 8.2, 3.4 Hz, H-3′), 5.27 (m, 1H, H-4′), 5.31 (t, 1H, J = 7.9 Hz, H-2′), 5.60 (d, 1H, J = 7.9 Hz, H-1′), 7.23 (d, 1H, J = 9.5 Hz, Ar-H), 7.26 (d, 1H, J = 9.5 Hz, Ar-H). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (COCH3), 20.8 (COCH3), 20.9 (COCH3), 56.9 (-OCH3), 57.2 (-OCH3), 61.2 (-OCH3), 65.3 (C-5′), 67.6 (C-4′), 69.2 (C-2′), 70.3 (C-3′), 81.8 (C-1′), 119.4, 120.6, 120.7, 122.1, 127.1 (C-2), 153.2, 153.7, 159.1 (C-3), 169.5 (COCH3), 169.8 (COCH3), 170.2 (COCH3), 178.3, 181.4. IR (CHCl3): 3054, 3005, 2941, 2841, 1747, 1661, 1597, 1570, 1478, 1463, 1435, 1412, 1372, 1335, 1272, 1250, 1185, 1159, 1105, 1087, 1060, 1022 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C24H25O12S 537.1072, found 537.1070.
2-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl-1-thio)-6,7-dichloro-3,5,8-trimethoxy-1,4-naphthoquinone (14a); yield 279 mg (82%), yellow solid, Rf 0.48 (A), m.p. 103–105 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.96 (s, 3H, COCH3), 2.01 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 3.67 (m, 1H, H-5′), 3.98 (s, 6H, 2 × -OCH3), 4.01 (dd, 1H, J = 12.6, 2.2 Hz, H-6′a), 4.15 (s, 3H, -OCH3), 4.18 (dd, 1H, J = 12.6, 4.7 Hz, H-6′b), 5.10 (m, 2H, H-2′, H-4′), 5.27 (t, 1H, J = 9.3 Hz, H-3′), 5.60 (d, 1H, J = 10.2 Hz, H-1′). 13C-NMR(125 MHz, CDCl3), δ: 20.5 (2 × COCH3), 20.6 (2 × COCH3), 61.3 (-OCH3), 61.8 (C-6′), 62.3 (-OCH3), 62.4 (-OCH3), 68.1 (C-4′), 71.1 (C-2′), 74.0 (C-3′), 76.0 (C-5′), 80.4 (C-1′), 124.1, 125.7, 127.6 (C-2), 136.2, 136.9, 152.6, 153.0, 158.3 (C-3), 169.3 (COCH3), 169.4 (COCH3), 170.1 (COCH3), 170.5 (COCH3), 176.4, 179.7. IR (CHCl3): 3050, 2944, 2857, 1756, 1670, 1581, 1547, 1459, 1440, 1380, 1326, 1304, 1241, 1207, 1194, 1030 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C27H27Cl2O14S 677.0504, found 677.0504.
2-(2,3,4,6-Tetra-O-acetyl-β-d-galactopyranosyl-1-thio)-6,7-dichloro-3,5,8-trimethoxy-1,4-naphthoquinone (14b); yield 302 mg (88.8%), orange solid, Rf 0.48 (A), m.p. 80–81 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.95 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 2.09 (s, 3H, COCH3), 2.14 (s, 3H, COCH3), 3.90 (m, 1H, H-5′), 3.98 (s, 6H, 2 × -OCH3), 4.03 (m, 2H, H-6′a, H-6′b), 4.16 (s, 3H, -OCH3), 5.11 (dd, 1H, J = 9.9, 3.5 Hz, H-3′), 5.30 (t, 1H, J = 10.0 Hz, H-2′), 5.42 (m, 1H, H-4′), 5.57 (d, 1H, J = 10.2 Hz, H-1′). 13C-NMR(125 MHz, CDCl3), δ: 20.5 (2 × COCH3), 20.6(COCH3), 20.7 (COCH3), 61.2 (C-6′), 61.7 (-OCH3), 62.3 (-OCH3), 62.4 (-OCH3), 67.2 (C-4′), 68.3 (C-2′), 71.9 (C-3′), 74.6 (C-5′), 81.3 (C-1′), 124.1, 125.6, 127.7 (C-2), 136.2, 136.9, 152.7, 153.0, 158.4 (C-3), 169.6 (COCH3), 170.0 (COCH3), 170.2 (COCH3), 170.3 (COCH3), 176.5, 179.7. IR (CHCl3): 3053, 3007, 2944, 2856, 1751, 1670, 1580, 1459, 1440, 1380, 1326, 1304, 1245, 1191, 1113, 1086, 1059, 1028 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C27H27Cl2O14S 677.0504, found 677.0501.
2-(2,3,4-Tri-O-acetyl-β-d-xylopyranosyl-1-thio)-6,7-dichloro-3,5,8-trimethoxy-1,4-naphthoquinone (14c); yield 263 mg (86.5%), dark orange solid, Rf 0.52 (A), m.p. 132–134 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.04 (s, 3H, COCH3), 2.07 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 3.36 (dd, 1H, J = 11.8, 8.6 Hz, H-5′a), 3.97 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 4.15 (s, 3H, -OCH3), 4.18 (dd, 1H, J = 11.8, 5.0 Hz, H-5′b), 4.95 (m, 1H, H-4′), 5.04 (t, 1H, J = 8.2 Hz, H-2′), 5.22 (t, 1H, J = 8.2 Hz, H-3′), 5.57 (d, 1H, J = 8.2 Hz, H-1′). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (3 × COCH3), 61.3 (-OCH3), 62.3 (2×-OCH3), 65.2 (C-5′), 68.5 (C-4′), 70.7 (C-2′), 71.9 (C-3′), 81.3 (C-1′), 124.1, 125.4, 127.6 (C-2), 136.2, 136.9, 152.8, 153.0, 159.1 (C-3), 169.4 (COCH3), 169.7 (COCH3), 169.9 (COCH3), 176.7, 179.7. IR (CHCl3): 3054, 3019, 3006, 2944, 2857, 1754, 1671, 1580, 1547, 1459, 1440, 1380, 1326, 1304, 1246, 1190, 1114, 1067, 1029 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C24H23Cl2O12S 605.0293, found 605.0291.
2-(2,3,4-Tri-O-acetyl-α-l-arabinopyranosyl-1-thio)-6,7-dichloro-3,5,8-trimethoxy-1,4-naphthoquinone (14d); yield 276 mg (90.8%), dark orange solid, Rf 0.50 (A), m.p. 79–81 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.07 (s, 3H, COCH3), 2.11 (s, 6H, 2 × COCH3), 3.63 (dd, 1H, J = 12.7, 1.9 Hz, H-5′a), 3.96 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 4.06 (dd, 1H, J = 12.7, 4.2 Hz, H-5′b), 4.16 (s, 3H, -OCH3), 5.16 (dd, 1H, J = 8.3, 3.4 Hz, H-3′), 5.28 (m, 1H, H-4′), 5.31 (t, 1H, J = 8.0 Hz, H-2′), 5.57 (d, 1H, J = 8.0 Hz, H-1′). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (COCH3), 20.8 (COCH3), 20.8 (COCH3), 61.3 (-OCH3), 62.3 (2 × -OCH3), 65.6 (C-5′), 67.5 (C-4′), 69.2 (C-2′), 70.3 (C-3′), 81.6 (C-1′), 124.1, 125.5, 128.0 (C-2), 136.1, 136.9, 152.8, 153.0, 158.9 (C-3), 169.4 (COCH3), 169.9 (COCH3), 170.2 (COCH3), 176.7, 179.8. IR (CHCl3): 3054, 3027, 3005, 2943, 2856, 1748, 1670, 1580, 1546, 1459, 1440, 1380, 1326, 1304, 1247, 1225, 1190, 1113, 1087, 1060, 1028 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C24H23Cl2O12S 605.0293, found 605.0289.
2-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl-1-thio)-8-hydroxy-3-methoxy-1,4-naphthoquinone (15a); yield 210 mg (73.9%), orange solid, Rf 0.46 (A), m.p. 158–161 °C. 1H-NMR (700 MHz, CDCl3): δ 1.93 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.09 (s, 3H, COCH3), 3.72 (m, 1H, H-5′), 4.08 (dd, 1H, J = 12.4, 2.0 Hz, H-6′a), 4.15 (dd, 1H, J = 12.4, 5.5 Hz, H-6′b), 4.29 (s, 3H, -OCH3), 5.08 (t, 1H, J = 9.7 Hz, H-4′), 5.11 (t, 1H, J = 10.1 Hz, H-2′), 5.27 (t, 1H, J = 9.3 Hz, H-3′), 5.50 (d, 1H, J = 10.1 Hz, H-1′), 7.26 (dd, 1H, J = 7.4, 1.2 Hz, H-7), 7.59 (dd, 1H, J = 8.0, 7.4 Hz, H-6), 7.61 (dd, 1H, J = 8.0, 1.2 Hz, H-5), 12.03 (s, 1H, C8OH). 13C-NMR(176 MHz, CDCl3): δ 20.4 (COCH3), 20.5 (COCH3), 20.6 (COCH3), 20.7 (COCH3), 62.1 (C-6′, -OCH3), 68.3 (C-4′), 71.3 (C-2′), 73.9 (C-3′), 76.0 (C-5′), 81.7 (C-1′), 114.3 (C-9), 119.7 (C-5), 125.0 (C-7), 126.0 (C-2), 131.3 (C-10), 135.8 (C-6), 160.5 (C-3), 161.2 (C-8), 169.3 (COCH3), 169.4 (COCH3), 170.2 (COCH3), 170.5 (COCH3), 178.4 (C-4), 187.7 (C-1). IR (CHCl3): 3053, 3007, 2953, 1756, 1669, 1630, 1580, 1559, 1458, 1369, 1313, 1250, 1191, 1162, 1077, 1050 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C25H25O13S 565.1021, found 565.1016.
2-(2,3,4,6-Tetra-O-acetyl-β-d-galactopyranosyl-1-thio)-8-hydroxy-3-methoxy-1,4-naphthoquinone (15b); yield 203 mg (71.4%), orange solid, Rf 0.46 (A), m.p. 190–193 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.91 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 2.15 (s, 3H, COCH3), 3.92 (m, 1H, H-5′), 4.06 (m, 2H, H-6′a, H-6′b), 4.30 (s, 3H, -OCH3), 5.10 (dd, 1H, J = 9.9, 3.5 Hz, H-3′), 5.32 (t, 1H, J = 10.0 Hz, H-2′), 5.43 (m, 1H, H-4′), 5.46 (d, 1H, J = 10.0 Hz, H-1′), 7.26 (dd, 1H, J = 7.7, 1.5 Hz, H-7), 7.58 (dd, 1H, J = 7.7, 7.5 Hz, H-6), 7.61 (dd, 1H, J = 7.5, 1.5 Hz, H-5), 12.04 (s, 1H, C8OH). 13C-NMR(125 MHz, CDCl3), δ: 20.4 (COCH3), 20.5 (COCH3), 20.6 (COCH3), 20.8 (COCH3), 61.6 (C-6′), 62.1 (-OCH3), 67.3 (C-4′), 68.4 (C-2′), 71.9 (C-3′), 74.8 (C-5′), 82.5 (C-1′), 114.3 (C-9), 119.6 (C-5), 125.0 (C-7), 126.2 (C-2), 131.4 (C-10), 135.8 (C-6), 160.5 (C-3), 161.3 (C-8), 169.6 (COCH3), 170.0 (COCH3), 170.2 (COCH3), 170.3 (COCH3), 178.5 (C-4), 187.7 (C-1). IR (CHCl3): 3055, 3019, 2954, 1751, 1669, 1630, 1580, 1559, 1458, 1441, 1370, 1312, 1249, 1193, 1162, 1080, 1053 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C25H25O13S 565.1021, found 565.1021.
2-(2,3,4-Tri-O-acetyl-β-d-xylopyranosyl-1-thio)-8-hydroxy-3-methoxy-1,4-naphthoquinone (15c); yield 205 mg (82.6%), orange solid, Rf 0.50 (A), m.p. 133–135 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.04 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 2.11 (s, 3H, COCH3), 3.41 (dd, 1H, J = 11.9, 8.1 Hz, H-5′a), 4.25 (dd, 1H, J = 11.9, 4.8 Hz, H-5′b), 4.29 (s, 3H, -OCH3), 4.95 (m, 1H, H-4′), 5.05 (t, 1H, J = 7.8 Hz, H-2′), 5.21 (t, 1H, J = 7.8 Hz, H-3′), 5.51 (d, 1H, J = 7.9 Hz, H-1′), 7.26 (dd, 1H, J = 8.0, 1.7 Hz, H-7), 7.57 (dd, 1H, J = 8.0, 7.5 Hz, H-6), 7.60 (dd, 1H, J = 7.5, 1.5 Hz, H-5), 12.05 (s, 1H, C8OH). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (3 × COCH3), 62.1 (-OCH3), 64.9 (C-5′), 68.3 (C-4′), 70.6 (C-2′), 71.5 (C-3′), 82.7 (C-1′), 114.4 (C-9), 119.6 (C-5), 125.0 (C-7), 126.0 (C-2), 131.4 (C-10), 135.8 (C-6), 161.3 (C-8), 161.4 (C-3), 169.4 (COCH3), 169.7 (COCH3), 169.8 (COCH3), 178.8 (C-4), 187.7 (C-1). IR (CHCl3): 3056, 2953, 1752, 1671, 1630, 1580, 1558, 1458, 1371, 1313, 1249, 1240, 1208, 1163, 1076 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C22H21O11S 493.0810, found 493.0805.
2-(2,3,4-Tri-O-acetyl-α-l-arabinopyranosyl-1-thio)-8-hydroxy-3-methoxy-1,4-naphthoquinone (15d); yield 197 mg (79.4%), orange solid, Rf 0.50 (A), m.p. 84–86 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.10 (s, 3H, COCH3), 2.12 (s, 6H, 2 × COCH3), 3.65 (dd, 1H, J = 12.5, 2.5 Hz, H-5′a), 4.14 (dd, 1H, J = 12.5, 4.9 Hz, H-5′b), 4.30 (s, 3H, -OCH3), 5.17 (dd, 1H, J = 7.9, 3.4 Hz, H-3′), 5.28 (m, 1H, H-4′), 5.32 (t, 1H, J = 7.5 Hz, H-2′), 5.48 (d, 1H, J = 7.5 Hz, H-1′), 7.26 (dd, 1H, J = 7.8, 1.6 Hz, H-7), 7.57 (dd, 1H, J = 7.8, 7.5 Hz, H-6), 7.60 (dd, 1H, J = 7.5, 1.6 Hz, H-5), 12.06 (s, 1H, C8OH). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (COCH3), 20.8 (COCH3), 20.9 (COCH3), 62.1 (-OCH3), 64.7 (C-5′), 67.2 (C-4′), 69.4 (C-2′), 70.0 (C-3′), 82.9 (C-1′), 114.4 (C-9), 119.6 (C-5), 125.0 (C-7), 126.5 (C-2), 131.3 (C-10), 135.8 (C-6), 161.2 (C-3), 161.3 (C-8), 169.4 (COCH3), 169.8 (COCH3), 170.1 (COCH3), 178.8 (C-4), 187.7 (C-1). IR (CHCl3): 3054, 3006, 1748, 1671, 1629, 1580, 1558, 1458, 1372, 1313, 1250, 1162, 1106, 1078, 1061 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C22H21O11S 493.0810, found 493.0808.
2-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl-1-thio)-5-hydroxy-3-methoxy-1,4-naphthoquinone (16a); yield 248 mg (87.3%), red solid, Rf 0.46 (A), m.p. 159–161 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.93 (s, 3H, COCH3), 2.01 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 3.73 (m, 1H, H-5′), 4.06 (dd, 1H, J = 12.4, 2.3 Hz, H-6′a), 4.16 (dd, 1H, J = 12.4, 5.2 Hz, H-6′b), 4.22 (s, 3H, -OCH3), 5.09 (dd, 1H, J = 10.1, 4.2 Hz, H-4′), 5.11 (dd, 1H, J = 10.1, 4.1 Hz, H-2′), 5.27 (t, 1H, J = 9.3 Hz, H-3′), 5.67 (d, 1H, J = 10.1 Hz, H-1′), 7.24 (dd, 1H, J = 8.1, 1.4 Hz, H-6), 7.59 (dd, 1H, J = 8.1, 7.5 Hz, H-7), 7.62 (dd, 1H, J = 7.5, 1.4 Hz, H-8), 11.72 (s, 1H, C5OH). 13C-NMR(125 MHz, CDCl3), δ: 20.5 (2 × COCH3), 20.6 (2 × COCH3), 62.0 (-OCH3, C-6′), 68.3 (C-4′), 71.2 (C-2′), 74.0 (C-3′), 75.9 (C-5′), 81.1 (C-1′), 114.1 (C-10), 119.7 (C-8), 124.4 (C-6), 130.1 (C-2), 132.3 (C-9), 136.5 (C-7), 158.6 (C-3), 161.8 (C-5), 169.3 (COCH3), 169.4 (COCH3), 170.1 (COCH3), 170.5 (COCH3), 181.3 (C-1), 183.5 (C-4). IR (CHCl3): 3050, 3004, 2950, 1756, 1661, 1636, 1579, 1560, 1458, 1369, 1240, 1228, 1212, 1192, 1046 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C25H25O13S 565.1021, found 565.1017.
2-(2,3,4,6-Tetra-O-acetyl-β-d-galactopyranosyl-1-thio)-5-hydroxy-3-methoxy-1,4-naphthoquinone (16b); yield 233 mg (82.0%), dark orange solid, Rf 0.46 (A), m.p. 83–86 °C. 1H-NMR (500 MHz, CDCl3), δ: 1.91 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 2.09 (s, 3H, COCH3), 2.15 (s, 3H, COCH3), 3.93 (m, 1H, H-5′), 4.05 (m, 2H, H-6′a, H-6′b), 4.23 (s, 3H, -OCH3), 5.11 (dd, 1H, J = 9.9, 3.4 Hz, H-3′), 5.31 (t, 1H, J = 10.0 Hz, H-2′), 5.43 (m, 1H, H-4′), 5.64 (d, 1H, J = 10.2 Hz, H-1′), 7.24 (dd, 1H, J = 8.2, 1.3 Hz, H-6), 7.59 (dd, 1H, J = 8.2, 7.5 Hz, H-7), 7.63 (dd, 1H, J = 7.5, 1.3 Hz, H-8), 11.72 (s, 1H, C5OH). 13C-NMR(125 MHz, CDCl3), δ: 20.5 (COCH3), 20.55 (COCH3′), 20.6 (COCH3), 20.7 (COCH3), 61.4 (C-6′), 62.0 (-OCH3), 67.3 (C-4′), 68.3 (C-2′), 71.9 (C-3′), 74.6 (C-5′), 81.9 (C-1′), 114.1 (C-10), 119.7 (C-8), 124.3 (C-6), 130.2 (C-2), 132.3 (C-9), 136.5 (C-7), 158.6 (C-3), 161.8 (C-5), 169.6 (COCH3), 170.0 (COCH3), 170.2 (COCH3), 170.3 (COCH3), 181.2 (C-1), 183.5 (C-4). IR (CHCl3): 3056, 3006, 1751, 1661, 1636, 1579, 1563, 1458, 1371, 1255, 1191, 1171, 1154, 1085, 1049 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C25H25O13S 565.1021, found 565.1019.
2-(2,3,4-Tri-O-acetyl-β-d-xylopyranosyl-1-thio)-5-hydroxy-3-methoxy-1,4-naphthoquinone (16c); yield 181 mg (73.0%), orange solid, Rf 0.50 (A), m.p. 135–137 °C. 1H-NMR (500 MHz, CDCl3), δ: 2.04 (s, 3H, COCH3), 2.07 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 3.40 (dd, 1H, J = 11.8, 8.4 Hz, H-5′a), 4.20 (dd, 1H, J = 11.8, 5.0 Hz, H-5′b), 4.22 (s, 3H, -OCH3), 4.96 (m, 1H, H-4′), 5.05 (t, 1H, J = 8.2 Hz, H-2′), 5.22 (t, 1H, J = 8.1 Hz, H-3′), 5.67 (d, 1H, J = 8.2 Hz, H-1′), 7.23 (dd, 1H, J = 8.1, 1.3 Hz, H-6), 7.59 (dd, 1H, J = 8.1, 7.5 Hz, H-7), 7.63 (dd, 1H, J = 7.5, 1.3 Hz, H-8), 11.71 (s, 1H, C5OH). 13C-NMR(125 MHz, CDCl3), δ: 20.7 (3 × COCH3), 62.1 (-OCH3), 65.1 (C-5′), 68.5 (C-4′), 70.6 (C-2′), 71.8 (C-3′), 82.0 (C-1′), 114.2 (C-10), 119.8 (C-8), 124.3 (C-6), 130.0 (C-2), 132.3 (C-9), 136.6 (C-7), 159.4 (C-3), 161.8 (C-5), 169.4 (COCH3), 169.7 (COCH3), 169.8 (COCH3), 181.3 (C-1), 183.7 (C-4). IR (CHCl3): 3053, 3007, 2949, 1754, 1661, 1637, 1579, 1563, 1458, 1371, 1248, 1192, 1171, 1070, 1048 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C22H21O11S 493.0810, found 493.0811.
2-(2,3,4-Tri-O-acetyl-α-l-arabinopyranosyl-1-thio)-5-hydroxy-3-methoxy-1,4-naphthoquinone (16d); yield 189 mg (76.2%), dark orange solid, Rf 0.50 (A), m.p. 97–99 °C. 1H-NMR(700 MHz, CDCl3), δ: 2.08 (s, 3H, COCH3), 2.11 (s, 3H, COCH3), 2.12 (s, 3H, COCH3), 3.66 (dd, 1H, J = 12.6, 2.3 Hz, H-5′a), 4.09 (dd, 1H, J = 12.6, 4.4 Hz, H-5′b), 4.23 (s, 3H, -OCH3), 5.16 (dd, 1H, J = 8.2, 3.4 Hz, H-3′), 5.29 (m, 1H, H-4′), 5.31 (t, 1H, J = 7.7 Hz, H-2′), 5.66 (d, 1H, J = 7.7 Hz, H-1′), 7.23 (dd, 1H, J = 8.3, 1.0 Hz, H-6), 7.59 (dd, 1H, J = 8.3, 7.5 Hz, H-7), 7.63 (dd, 1H, J = 7.5, 1.0 Hz, H-8), 11.72 (s, 1H, C5OH). 13C-NMR (176 MHz, CDCl3), δ: 20.7 (COCH3), 20.8 (COCH3), 20.9 (COCH3), 62.0 (-OCH3), 65.3 (C-5′), 67.4 (C-4′), 69.2 (C-2′), 70.2 (C-3′), 82.2 (C-1′), 114.2 (C-10), 119.8 (C-8), 124.3 (C-6), 130.5 (C-2), 132.3 (C-9), 136.6 (C-7), 159.3 (C-3), 161.8 (C-5), 169.5 (COCH3), 169.8 (COCH3), 170.2 (COCH3), 181.4 (C-1), 183.8 (C-4). IR (CHCl3): 3056, 2947, 1748, 1661, 1636, 1579, 1562, 1457, 1371, 1240, 1193, 1170, 1105, 1087, 1066, 1024 cm−1. HRMS (ESI): m/z [M − H]− calcd. for C22H21O11S 493.0810, found 493.0807.
3.2.2. General Procedure for Cyclization of Acetylated Thioglycosides to Tetracyclic Conjugates 17a–d–20a–d
Acetylated thioglycoside, 13a–d–16a–d (0.25 mM), was suspended in 15 mL of dried MeOH and 0.9 mL MeONa solution (0.5 N) was added. The mixture was stirred at room temperature for 1 h until TLC analysis indicated complete consumption of initial thioglycoside. During the reaction, the formation of a new polar compound precipitate was also observed. The reaction mixture was acidified with 0.25 mL HCl solution (2 N) and the precipitate was filtered off, washed with water, cold MeOH, and gave high purity quinone-tioglycosidic conjugates 17a–d–20a–d.
(2R,3S,4S,4aR,12aS)-3,4-Dihydroxy-2-hydroxymethyl-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (17a); yield 88 mg (86.1%), red solid, Rf 0.35 (C), m.p. 332–335 °C27. 1H-NMR(700 MHz, DMSO-d6), δ: 3.30 (m, 1H, H-3), 3.47 (m, 3H, H-2, H-4a, H-13a), 3.57 (m, 1H, H-4), 3.74 (m,1H, H-13b), 3.84 (s, 3H, -OCH3), 3.85 (s, 3H, -OCH3), 4.72 (br s, 1H, C13OH), 4.92 (d, 1H, J = 8.3 Hz, H-12a), 5.37 (br s, 1H, C3OH), 5.58 (br s, 1H, C4OH), 7.52 (d, 1H, J = 9.6 Hz, Ar-H), 7.54 (d, 1H, J = 9.6 Hz, Ar-H). 13C-NMR (176 MHz, DMSO-d6), δ: 56.7 (-OCH3), 56.8 (-OCH3), 60.8 (C-13), 70.5 (C-3), 73.7 (C-12a), 73.9 (C-4), 79.2 (C-4a), 82.1 (C-2), 118.6, 118.9, 121.7, 122.0, 122.4, 149.7, 153.3, 153.8, 174.7, 179.9. IR (KBr): 3444, 1638, 1614, 1561, 1476, 1405, 1266, 1181, 1075, 935 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C18H18NaO9S 433.0564, found 433.0562.
(2R,3R,4S,4aR,12aS)-3,4-Dihydroxy-2-hydroxymethyl-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (17b); yield 95 mg (93%), orange solid, Rf 0.30 (C), m.p. > 350 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.55 (m, 2H, H-13), 3.72 (t, 1H, J = 6.0 Hz, H-2), 3.77 (m, 2H, H-4, H-4a), 3.83 (m, 1H, H-3), 3.84 (s, 3H, -OCH3), 3.85 (s, 3H, -OCH3), 4.74 (t, 1H, J = 5.6 Hz, C13OH), 4.88 (d, 1H, J = 7.6 Hz, H-12a), 4.89 (d, 1H, J = 4.6 Hz, C3OH), 5.31 (d, 1H, J = 6.3 Hz, C4OH), 7.52 (d, 1H, J = 9.7 Hz, Ar-H), 7.55 (d, 1H, J = 9.7 Hz, Ar-H). 13C-NMR (125 MHz, DMSO-d6), δ: 56.7 (-OCH3), 56.8 (-OCH3), 60.5 (C-13), 69.4 (C-3), 70.4 (C-4), 74.4 (C-12a), 77.7 (C-4a), 80.6 (C-2), 118.6, 118.9, 121.7, 122.0, 122.4, 150.3, 153.3, 153.8, 174.4, 179.8. IR (KBr): 3487, 3289, 3016, 2968, 2935, 2838, 1649, 1611, 1581, 1562, 1476, 1434, 1407, 1384, 1349, 1325, 1267, 1213, 1196, 1180, 1110, 1082, 1056, 1041, 1019, 1008, 936, 904, 873, 824, 803, 755 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C18H18NaO9S 433.0564, found 433.0565.
(3R,4S,4aR,12aS)-3,4-Dihydroxy-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (17c); yield 80 mg (83.9%), orange solid, Rf 0.47 (C), m.p. 329–332 °C. 1H-NMR(700 MHz, DMSO-d6), δ: 3.37 (m, 1H, H-2a), 3.48 (m, 1H, H-4a), 3.52 (m, 2H, H-3, H-4), 3.84 (s, 3H, -OCH3), 3.85 (s, 3H, -OCH3), 3.91 (dd, 1H, J = 11.1, 4.8 Hz, H-2b), 4.85 (d, 1H, J = 8.1 Hz, H-12a), 5.38 (d, 1H, J = 4.8 Hz, C3OH), 5.61 (d, 1H, J = 5.3 Hz, C4OH), 7.52 (d, 1H, J = 9.6 Hz, Ar-H), 7.54 (d, 1H, J = 9.6 Hz, Ar-H). 13C-NMR (176 MHz, DMSO-d6), δ: 56.7 (-OCH3), 56.8 (-OCH3), 70.0 (C-3), 70.3 (C-2), 74.1 (C-4), 74.6 (C-12a), 79.2 (C-4a), 118.6, 118.9, 121.7, 122.1, 122.3, 149.7, 153.4, 153.9, 174.6, 179.8. IR (KBr): 3502, 3470, 3296, 3013, 2981, 2939, 2877, 2836, 1661, 1636, 1611, 1581, 1562, 1478, 1459, 1431, 1408, 1359, 1281, 1267, 1253, 1223, 1196, 1164, 1125, 1095, 1060, 1042, 1023, 975, 935, 887, 818, 797, 755, 718 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C17H16NaO8S 403.0458, found 403.0459.
(3S,4S,4aR,12aS)-3,4-Dihydroxy-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (17d); yield 88 mg (92.3%), yellow solid, Rf 0.43 (C), m.p. 317–320 °C. 1H-NMR(700 MHz, DMSO-d6), δ: 3.77 (m, 3H, H-2a, H-4, H-4a), 3.84 (m, 1H, H-3), 3.85 (s, 3H, -OCH3), 3.86 (s, 3H, -OCH3), 3.88 (dd, 1H, J = 12.2, 1.7 Hz, H-2b), 4.82 (d, 1H, J = 7.7 Hz, H-12a), 5.02 (d, 1H, J = 4.1 Hz, C3OH), 5.31 (d, 1H, J = 6.6 Hz, C4OH), 7.52 (d, 1H, J = 9.6 Hz, Ar-H), 7.55 (d, 1H, J = 9.6 Hz, Ar-H). 13C-NMR (176 MHz, DMSO-d6), δ: 56.7 (-OCH3), 56.8 (-OCH3), 69.4 (C-3), 69.8 (C-4), 71.4 (C-2), 74.8 (C-12a), 77.6 (C-4a), 118.6, 118.9, 121.7, 122.0, 122.4, 150.2, 153.4, 153.8, 174.6, 179.8. IR (KBr): 3496, 3449, 3094, 3012, 2979, 2932, 2864, 2837, 1659, 1642, 1613, 1479, 1459, 1433, 1409, 1352, 1338, 1325, 1280, 1259, 1206, 1188, 1166, 1123, 1097, 1080, 1069, 1048, 1022, 1007, 955, 934, 910, 866, 833, 815, 800, 747 cm−1. HRMS (ESI, m/z): [M + Na]+ calcd. for C17H16NaO8S 403.0458, found 403.0453.
(2R,3S,4S,4aR,12aS)-8,9-Dichloro-3,4-dihydroxy-2-hydroxymethyl-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (18a); yield 100 mg (83.4%), orange solid, Rf 0.51 (C), m.p. 222–224 °C27. 1H-NMR(500 MHz, DMSO-d6), δ: 3.32 (m, 1H, H-3), 3.50 (m, 2H, H-2, H-13a), 3.54 (m, 1H, H-4a), 3.60 (m, 1H, H-4), 3.75 (m, 1H, H-13b), 3.82 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 4.74 (t, 1H, J = 5.3 Hz, C13OH), 4.96 (d, 1H, J = 8.1 Hz, H-12a), 5.40 (d, 1H, J = 5.7 Hz, C3OH), 5.67 (d, 1H, J = 5.8 Hz, C4OH). 13C-NMR (125 MHz, DMSO-d6), δ: 60.7 (C-13), 61.5 (2 × -OCH3), 70.4 (C-3), 73.6 (C-12a), 73.9 (C-4), 79.3 (C-4a), 82.2 (C-2), 123.1, 123.4, 123.9, 135.1, 135.3, 149.9, 152.3, 152.9, 173.4, 178.9. IR (KBr): 3432, 2941, 1653, 1603, 1625, 1458, 1381, 1333, 1275, 1209, 1131, 1025, 951 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C18H16Cl2NaO9S 500.9784, found 500.9784.
(2R,3R,4S,4aR,12aS)-8,9-Dichloro-3,4-dihydroxy-2-(hydroxymethyl)-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (18b); yield 98 mg (81.7%), orange solid, Rf 0.47 (C), m.p. 276–279 °C. 1H-NMR(700 MHz, DMSO-d6), δ: 3.56 (m, 2H, H-13), 3.75 (t, 1H, J = 6.0 Hz, H-2), 3.79 (dd, 1H, J = 9.6, 3.3 Hz, H-4), 3.82 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 3.85 (m, 2H, H-3, H-4a), 4.80 (br s, 2H, 2 × OH), 4.92 (d, 1H, J = 7.9 Hz, H-12a), 5.40 (br s, 1H, OH). 13C-NMR (176 MHz, DMSO-d6), δ: 60.5 (C-13), 61.5 (2 × -OCH3), 69.5 (C-3), 70.4 (C-4), 74.3 (C-12a), 77.9 (C-4a), 80.8 (C-2), 123.2, 123.4, 123.9, 135.1, 135.2, 150.5, 152.3, 152.9, 173.4, 178.9. IR (KBr): 3413, 2940, 2855, 1652, 1603, 1542, 1524, 1457, 1380, 1336, 1274, 1236, 1207, 1121, 1090, 1024, 945, 876, 839, 805, 762 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C18H16Cl2NaO9S 500.9784, found 500.9783.
(3R,4S,4aR,12aS)-8,9-Dichloro-3,4-dihydroxy-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (18c); yield 102 mg (90.7%), orange solid, Rf 0.59 (C), m.p. 277–279 °C. 1H-NMR(700 MHz, DMSO-d6), δ: 3.40 (m, 1H, H-2a), 3.54 (m, 3H, H-4a, H-4, H-3), 3.81 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 3.93 (dd, 1H, J = 11.2, 4.6 Hz, H-2b), 4.90 (d, 1H, J = 7.5 Hz, H-12a), 5.42 (d, 1H, J = 4.4 Hz, C3OH), 5.72 (d, 1H, J = 4.9 Hz, C4OH). 13C-NMR (176 MHz, DMSO-d6), δ: 61.5 (2 × -OCH3), 69.9 (C-3), 70.4 (C-2), 74.0 (C-4), 74.5 (C-12a), 79.4 (C-4a), 123.0, 123.4, 123.8, 135.1, 135.3, 149.9, 152.3, 152.9, 173.4, 178.9. IR (KBr): 3482, 3385, 3003, 2938, 2880, 2855, 1651, 1604, 1544, 1524, 1458, 1382, 1330, 1307, 1272, 1237, 1225, 1208, 1124, 1090, 1074, 1061, 1054, 1024, 975, 948, 898, 869, 838, 816, 802, 763, 741 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C17H14Cl2NaO8S 470.9679, found 470.9673.
(3S,4S,4aR,12aS)-8,9-Dichloro-3,4-dihydroxy-7,10-dimethoxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (18d); yield 97 mg (86.3%), orange solid, Rf 0.56 (C), m.p. 286–289 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.80 (m, 2H, H-2a, H-4), 3.82 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 3.85 (m, 2H, H-3, H-4a), 3.90 (dd, 1H, J = 12.2, 1.7 Hz, H-2b), 4.86 (d, 1H, J = 8.1 Hz, H-12a), 5.05 (br s, 1H, C3OH), 5.40 (br s, 1H, C4OH). 13C-NMR (125 MHz, DMSO-d6), δ: 61.5 (2 × -OCH3), 69.4 (C-3), 69.8 (C-4), 71.5 (C-2), 74.7 (C-12a), 77.8 (C-4a), 123.2, 123.4, 123.9, 135.1, 135.2, 150.4, 152.3, 152.9, 173.4, 178.9. IR (KBr): 3391, 2980, 2940, 2856, 1654, 1603, 1543, 1525, 1458, 1381, 1334, 1305, 1272, 1237, 1211, 1128, 1092, 1048, 1026, 967, 944, 913, 876, 839, 805, 757 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C17H14Cl2NaO8S 470.9679, found 470.9675.
(2R,3S,4S,4aR,12aS)-3,4,10-Trihydroxy-2-hydroxymethyl-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (19a); yield 86 mg (93.7%), orange solid, Rf 0.56 (C), decomposition > 342 °C. 1H-NMR(700 MHz, DMSO-d6), δ: 3.32 (m, 1H, H-3), 3.50 (m, 2H, H-2, H-13a), 3.60 (m, 2H, H-4, H-4a), 3.75 (m, 1H, H-13b), 4.74 (t, 1H, J = 5.5 Hz, C13OH), 5.01 (d, 1H, J = 7.7 Hz, H-12a), 5.41 (d, 1H, J = 5.8 Hz, C3OH), 5.69 (d, 1H, J = 5.7 Hz, C4OH), 7.30 (dd, 1H, J = 8.4, 0.7 Hz, H-9), 7.54 (dd, 1H, J = 7.4, 0.7 Hz, H-7), 7.70 (dd, 1H, J = 8.4, 7.4 Hz, H-8), 11.52 (s, 1H, C10OH). 13C-NMR (176 MHz, DMSO-d6), δ: 60.7 (C-13), 70.4 (C-3), 73.5 (C-12a), 73.9 (C-4), 79.4 (C-4a), 82.3 (C-2), 113.8 (C-10a), 119.2 (C-7), 122.9 (C-11a), 124.2 (C-9), 130.8 (C-6a), 136.5 (C-8), 151.0 (C-5a), 159.8 (C-10), 175.1 (C-6), 186.1 (C-11). IR (KBr): 3464, 3351, 3233, 2955, 2881, 1646, 1616, 1580, 1517, 1477, 1455, 1416, 1383, 1356, 1324, 1297, 1280, 1252, 1229, 1219, 1194, 1165, 1148, 1133, 1092, 1055, 1037, 1000, 973, 889, 863, 836, 828, 816, 755, 728 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C16H14NaO8S 389.0302, found 389.0300.
(2R,3R,4S,4aR,12aS)-3,4,10-Trihydroxy-2-hydroxymethyl-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano-[2,3-e][2,5]oxathiine-6,11-dione (19b); yield 75 mg (81.7%), dark orange solid, Rf 0.52 (C), decompose above > 346 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.57 (m, 2H, H-13), 3.76 (t, 1H, J = 6.0 Hz, H-2), 3.80 (m, 1H, H-4), 3.85 (m, 1H, H-3), 3.90 (t, 1H, J = 9.5, 8.0 Hz, H-4a), 4.77 (t, 1H, J = 5.6 Hz, C13OH), 4.96 (d, 1H, J = 4.8 Hz, C3OH), 4.98 (d, 1H, J = 8.0 Hz, H-12a), 5.43 (d, 1H, J = 6.8 Hz, C4OH), 7.30 (dd, 1H, J = 8.4, 0.9 Hz, H-9), 7.54 (dd, 1H, J = 7.4, 0.9 Hz, H-7), 7.70 (dd, 1H, J = 8.4, 7.4 Hz, H-8), 11.53 (s, 1H, C10OH). 13C-NMR (125 MHz, DMSO-d6), δ: 60.5 (C-13), 69.5 (C-3), 70.4 (C-4), 74.1 (C-12a), 78.1 (C-4a), 80.8 (C-2), 113.8 (C-10a), 119.2 (C-7), 122.9 (C-11a), 124.2 (C-9), 130.8 (C-6a), 136.5 (C-8), 151.5 (C-5a), 159.8 (C-10), 175.1 (C-6), 186.1 (C-11). IR (KBr): 3456, 3338, 2984, 2938, 2888, 1644, 1616, 1580, 1476, 1456, 1431, 1401, 1384, 1327, 1296, 1279, 1250, 1208, 1165, 1136, 1105, 1085, 1056, 1024, 979, 903, 879, 860, 833, 813, 754, 734, 690 cm−1. HRMS (ESI, m/z): [M + Na]+ calcd. for C16H14NaO8S 389.0302, found 389.0295.
(3R,4S,4aR,12aS)-3,4,10-Trihydroxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (19c); yield 76 mg (90.1%), orange solid, Rf 0.67 (C), m.p. 348–350 °C with decomposition. 1H-NMR(700 MHz, DMSO-d6), δ: 3.41 (m, 1H, H-2), 3.55 (m, 2H, H-3, H-4), 3.60 (m, 1H, H-4a), 3.94 (dd, 1H, J = 11.1, 4.7 Hz, H-2′), 4.95 (d, 1H, J = 8.0 Hz, C-12a), 5.42 (d, 1H, J = 4.7 Hz, C3OH), 5.73 (d, 1H, J = 5.3 Hz, C4OH), 7.30 (dd, 1H, J = 8.4, 0.9 Hz, H-9), 7.54 (dd, 1H, J = 7.4, 0.9 Hz, H-7), 7.70 (dd, 1H, J = 8.4, 7.4 Hz, H-8), 11.51 (s, 1H, C10OH). 13C-NMR (176 MHz, DMSO-d6), δ: 70.0 (C-3), 70.4 (C-2), 74.0 (C-4), 74.3 (C-12a), 79.4 (C-4a), 113.8 (C-10a), 119.2 (C-7), 122.8 (C-11a), 124.2 (C-9), 130.8 (C-6a), 136.6 (C-8), 151.0 (C-5a), 159.8 (C-10), 175.1 (C-6), 186.1 (C-11). IR (KBr): 3381, 3300, 2940, 2899, 2867, 1652, 1625, 1621, 1581, 1516, 1475, 1462, 1454, 1417, 1378, 1323, 1306, 1295, 1239, 1222, 1200, 1168, 1146, 1136, 1074, 1056, 1044, 1035, 1002, 976, 962, 900, 868, 833, 818, 789, 756 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C15H12NaO7S 359.0196, found 359.0197.
(3S,4S,4aR,12aS)-3,4,10-Trihydroxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (19d); yield 78 mg (92.5%), orange solid, Rf 0.62 (C), m.p. 319–321 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.79 (m, 2H, H-4, H-2a), 3.86 (m, 1H, H-3), 3.90 (m, 2H, H-4a, H-2b), 4.91 (d, 1H, J = 8.0 Hz, H-12a), 5.10 (d, 1H, J = 4.0 Hz, C3OH), 5.44 (d, 1H, J = 7.0 Hz, C4OH), 7.30 (dd, 1H, J = 8.4, 1.0 Hz, H-9), 7.53 (dd, 1H, J = 7.5, 1.0 Hz, H-7), 7.70 (dd, 1H, J = 8.4, 7.5 Hz, H-8), 11.54 (s, 1H, C10OH). 13C-NMR (125 MHz, DMSO-d6), δ: 69.4 (C-3), 69.7 (C-4), 71.5 (C-2), 74.5 (C-12a), 77.9 (C-4a), 113.8 (C-10a), 119.2 (C-7), 122.9 (C-11a), 124.2 (C-9), 130.7 (C-6a), 136.5 (C-8), 151.4 (C-5a), 159.8 (C-10), 175.1 (C-6), 186.1 (C-11). IR (KBr): 3511, 3196, 2987, 2925, 2892, 1654, 1624, 1578, 1475, 1463, 1444, 1378, 1356, 1333, 1308, 1279, 1237, 1177, 1164, 1139, 1109, 1084, 1071, 1022, 1004, 982, 938, 911, 837, 861, 835, 815, 754 cm−1. HRMS (ESI, m/z): [M + Na]+ calcd. for C15H12NaO7S 359.0196, found 359.0192.
(2R,3S,4S,4aR,12aS)-3,4,7-Trihydroxy-2-(hydroxymethyl)-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano-[2,3-e][2,5]oxathiine-6,11-dione (20a); yield 80 mg (87.1%), orange solid, Rf 0.56 (C), m.p. 274–276 °C. 1H-NMR(700 MHz, DMSO-d6), δ: 3.33 (m, 1H, H-3), 3.50 (m, 2H, H-2, H-13a), 3.60 (m, 2H, H-4, H-4a), 3.75 (m, 1H, H-13b), 4.73 (t, 1H, J = 5.6 Hz, C13OH), 5.01 (d, 1H, J = 7.7 Hz, H-12a), 5.41 (d, 1H, J = 5.7 Hz, C3OH), 5.68 (d, 1H, J = 5.7 Hz, C4OH), 7.32 (dd, 1H, J = 8.5, 0.9 Hz, H-8), 7.51 (dd, 1H, J = 7.4, 0.9 Hz, H-10), 7.70 (dd, 1H, J = 8.5, 7.4 Hz, H-9), 11.72 (s, 1H, C7OH). 13C-NMR (176 MHz, DMSO-d6), δ: 60.7 (C-13), 70.4 (C-3), 73.6 (C-12a), 73.9 (C-4), 79.2 (C-4a), 82.2 (C-2), 113.4 (C-6a), 118.7 (C-10), 124.1 (C-11a), 124.5 (C-8), 131.2 (C-10a), 136.5 (C-9), 150.0 (C-5a), 160.7 (C-7), 180.4 (C-11), 180.5 (C-6). IR (KBr): 3493, 3474, 3415, 3365, 3264, 2959, 2931, 2892, 1658, 1628, 1585, 1481, 1457, 1403, 1457, 1380, 1296, 1280, 1246, 1223, 1198, 1156, 1143, 1124, 1096, 1077, 1048, 1035, 1009, 952, 895, 869, 834, 816, 796, 747 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C16H14NaO8S 389.0302, found 389.0297.
(2R,3R,4S,4aR,12aS)-3,4,7-Trihydroxy-2-hydroxymethyl-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (20b); yield 75 mg (81.7%), orange solid, Rf 0.52 (C), m.p. 293–295 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.56 (m, 2H, H-13), 3.76 (t, 1H, J = 6.0 Hz, H-2), 3.80 (dd, 1H, J = 9.5, 3.2 Hz, H-4), 3.85 (dd, 1H, J = 3.3, 1.2 Hz, H-3), 3.89 (d, 1H, J = 9.5, 7.9 Hz, H-4a), 4.83 (br s, 3H, C3OH, C4OH, C13OH), 4.97 (d, 1H, J = 7.9 Hz, H-12a), 7.32 (dd, 1H, J = 8.5, 1.0 Hz, H-8), 7.51 (dd, 1H, J = 7.5, 1.0 Hz, H-10), 7.70 (dd, 1H, J = 8.5, 7.5 Hz, H-9), 11.72 (s, 1H, C7OH). 13C-NMR (125 MHz, DMSO-d6), δ: 60.5 (C-13), 69.5 (C-3), 70.4 (C-4), 74.2 (C-12a), 77.9 (C-4a), 80.8 (C-2), 113.4 (C-6a), 118.7 (C-10), 124.1 (C-11a), 124.5 (C-8), 131.3 (C-10a), 136.5 (C-9), 150.6 (C-5a), 160.7 (C-7), 180.4 (C-11), 180.5 (C-6). IR (KBr): 3412, 2941, 1627, 1581, 1475, 1454, 1385, 1297, 1282, 1241, 1208, 1192, 1155, 1129, 1074, 1049, 986, 954, 937, 870, 832, 813, 745, 698 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C16H14NaO8S 389.0302, found 389.0296.
(3R,4S,4aR,12aS)-3,4,7-Trihydroxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (20c); yield 70 mg (83.0%), orange solid, Rf 0.67 (C), m.p. 334–336 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.41 (m, 1H, H-2a), 3.56 (m, 3H, H-3, H-4, H-4a), 3.94 (dd, 1H, J = 11.0, 4.7 Hz, H-2b), 4.95 (d, 1H, J = 7.5 Hz, H-12a), 5.42 (d, 1H, J = 4.8 Hz, C3OH), 5.73 (d, 1H, J = 5.1 Hz, C4OH), 7.32 (dd, 1H, J = 8.5, 1.0 Hz, H-8), 7.51 (dd, 1H, J = 7.5, 1.0 Hz, H-10), 7.70 (dd, 1H, J = 8.5, 7.5 Hz, H-9), 11.71 (s, 1H, C7OH). 13C-NMR (125 MHz, DMSO-d6), δ: 69.4 (C-3), 69.7 (C-4), 71.5 (C-2), 74.5 (C-12a), 77.9 (C-4a), 113.8 (C-10a), 119.2 (C-7), 122.9 (C-11a), 124.2 (C-9), 130.7 (C-6a), 136.5 (C-8), 151.4 (C-5a), 159.8 (C-10), 180.4 (C-11), 180.5 (C-6). IR (KBr): 3401, 2887, 1653, 1622, 1579, 1476, 1454, 1407, 1390, 1297, 1277, 1251, 1220, 1207, 1194, 1156, 1134, 1090, 1063, 1048, 1008, 976, 952, 902, 840, 831, 818, 745 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C15H12NaO7S 359.0196, found 359.0194.
(3S,4S,4aR,12aS)-3,4,7-Trihydroxy-3,4,4a,12a-tetrahydro-2H-naphtho[2,3-b]pyrano[2,3-e][2,5]oxathiine-6,11-dione (20d); yield 82 mg (97.3%), dark orange solid, Rf 0.62 (C), m.p. 284–286 °C. 1H-NMR(500 MHz, DMSO-d6), δ: 3.79 (dd, 1H, J = 12.7, 3.4 Hz, H-2a), 3.80 (m, 1H, H-3), 3.86 (m, 1H, H-4), 3.89 (t, 1H, J = 8.0 Hz, H-4a), 3.91 (dd, 1H, J = 12.7, 1.8 Hz, H-2b), 4.90 (d, 1H, J = 8.0 Hz, H-12a), 5.08 (d, 1H, J = 4.0 Hz, C3OH), 5.41 (d, 1H, J = 6.9 Hz, C4OH), 7.32 (dd, 1H, J = 8.4, 1.0 Hz, H-8), 7.51 (dd, 1H, J = 7.5, 1.0 Hz, H-10), 7.69 (dd, 1H, J = 8.4, 7.5 Hz, H-9), 11.72 (s, 1H, C7OH). 13C-NMR (125 MHz, DMSO-d6), δ: 69.4 (C-3), 69.7 (C-4), 71.5 (C-2), 74.6 (C-12a), 77.7 (C-4a), 113.4 (C-6a), 118.7 (C-10), 124.1 (C-11a), 124.5 (C-8), 131.2 (C-10a), 136.4 (C-9), 150.5 (C-5a), 160.6 (C-7), 180.3 (C-11), 180.4 (C-6). IR (KBr): 3541, 3268, 3167, 2998, 2936, 1648, 1627, 1621, 1618, 1581, 1474, 1453, 1405, 1384, 1330, 1299, 1284, 1245, 1215, 1191, 1173, 1153, 1115, 1088, 1068, 1049, 1037, 1007, 950, 915, 881, 869, 841, 831, 812, 790, 744, 729 cm−1. HRMS (ESI): m/z [M + Na]+ calcd. for C15H12NaO7S 359.0196, found 359.0191.