Synthesis and Biological Studies on Dinuclear Gold(I) Complexes with Di-(N-Heterocyclic Carbene) Ligands Functionalized with Carbohydrates
Abstract
:1. Introduction
2. Results and Discussion
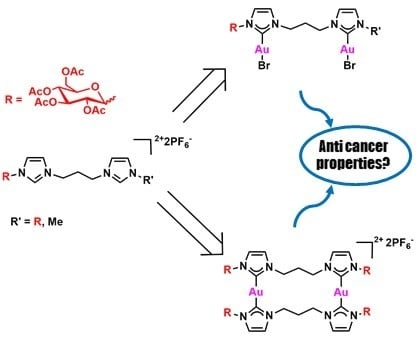
2.1. Synthesis of the Bis(Imidazolium) Salts
2.2. Synthesis of the [Au2Br2L] Complexes
2.3. Synthesis of the [Au2L2](PF6)2 Complexes
2.4. Reactivity of the Gold(I) Complex 3 toward Oxidative Addition of Halogens
2.5. Biological Activity of the Gold(I) Complexes
3. Materials and Methods
3.1. General Comments
3.2. Synthesis of the Bis(Imidazolium) Salts
3.2.1. Synthesis of the Bis(Imidazolium) Salt L1∙2HPF6
3.2.2. Synthesis of the Bis(Imidazolium) Salt L2∙2HPF6
3.3. General Procedure for the Synthesis of Complexes [Au2Br2L]
3.4. Synthesis of the Complex [Au2L22](PF6)2, 3
3.5. Reactivity of Complex 3 in Halogen Oxidative Addition: Characterization of the Dinuclear Au(III) Complexes [Au2L22X4](PF6)2, 4 and 5
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin−DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Diez-Gonzalez, S. N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, 2nd ed.; RSC Catalysis Series; RSC: Cambridge, UK, 2017. [Google Scholar]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Huynh, H.V. The Organometallic Chemistry of N-heterocyclic Carbenes; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2017. [Google Scholar]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903. [Google Scholar] [CrossRef]
- Oehninger, L.; Rubbiani, R.; Ott, I. N-Heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans. 2013, 42, 3269–3284. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Update on metal N-heterocyclic carbene complexes as potential anti-tumor metallodrugs. Co-ord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Teyssot, M.-L.; Jarrousse, A.-S.; Manin, M.; Chevry, A.; Roche, S.; Norre, F.; Beaudoin, C.; Morel, L.; Boyer, D.; Mahiou, R.; et al. Metal-NHC complexes: A survey of anti-cancer properties. Dalton Trans. 2009, 6894–6902. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Tong, K.-C.; Hu, D.; Wan, P.-K.; Lok, C.-N.; Che, C.-M. Anti-cancer gold, platinum and iridium compounds with porphyrin and/or N-heterocyclic carbene ligand(s). Adv. Inorg. Chem. 2020, 75, 87–119. [Google Scholar] [CrossRef]
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. Adv. Inorg. Chem. 2020, 75, 121–148. [Google Scholar] [CrossRef]
- Marzano, C.; Gandin, V.; Folda, A.; Scutari, G.; Bindoli, A.; Rigobello, M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free. Radic. Biol. Med. 2007, 42, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, B.; Correia, J.D.G.; Kühn, F.E. Medicinal Applications of Gold(I/III)-Based Complexes Bearing N-Heterocyclic Carbene and Phosphine Ligands. J. Organomet. Chem. 2018, 866, 153–164. [Google Scholar] [CrossRef]
- Baker, M.V.; Barnard, P.J.; Berners-Price, S.J.; Brayshaw, S.K.; Hickey, J.L.; Skelton, B.W.; White, A.H. Synthesis and structural characterisation of linear Au(I) N-heterocyclic carbene complexes: New analogues of the Au(I) phosphine drug Auranofin. J. Organomet. Chem. 2005, 690, 5625–5635. [Google Scholar] [CrossRef]
- Boselli, L.; Ader, I.; Carraz, M.; Hemmert, C.; Cuvillier, O.; Gornitzka, H. Synthesis, structures, and selective toxicity to cancer cells of gold(I) complexes involving N-heterocyclic carbene ligands. Eur. J. Med. Chem. 2014, 85, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cucciolito, M.E.; Trinchillo, M.; Iannitti, R.; Palumbo, R.; Tesauro, D.; Tuzi, A.; Ruffo, F.; D’Amora, A. Sugar-Incorporated N-Heterocyclic-Carbene-Containing Gold(I) Complexes: Synthesis, Characterization, and Cytotoxic Evaluation. Eur. J. Inorg. Chem. 2017, 4955–4961. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; D’Amora, A.; De Feo, G.; Ferraro, G.; Giorgio, A.; Petruk, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-Coordinate Platinum(II) Compounds Containing Sugar Ligands: Synthesis, Characterization, Cytotoxic Activity, and Interaction with Biological Macromolecules. Inorg. Chem. 2018, 57, 3133–3143. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; Bossa, F.D.L.; Esposito, R.; Ferraro, G.; Iadonisi, A.; Petruk, G.; D’Elia, L.; Romanetti, C.; Traboni, S.; Tuzi, A.; et al. C-Glycosylation in platinum-based agents: A viable strategy to improve cytotoxicity and selectivity. Inorg. Chem. Front. 2018, 5, 2921–2933. [Google Scholar] [CrossRef]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Imbimbo, P.; Petruk, G.; Ferraro, G.; Pinto, V.; Tuzi, A.; Monti, D.M.; Merlino, A.; et al. A highly efficient and selective antitumor agent based on a glucoconjugated carbene platinum(II) complex. Dalton Trans. 2019, 48, 7794–7800. [Google Scholar] [CrossRef]
- Zhao, W.; Ferro, V.; Baker, M.V. Carbohydrate–N-heterocyclic carbene metal complexes: Synthesis, catalysis and biological studies. Coord. Chem. Rev. 2017, 339, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, A.; Amoresano, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Iacobucci, I.; Imbimbo, P.; Lucignano, R.; Melchiorre, M.; Monti, M.; et al. Pt(II) versus Pt(IV) in Carbene Glycoconjugate Antitumor Agents: Minimal Structural Variations and Great Performance Changes. Inorg. Chem. 2020, 59, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Day, D. Mitochondrial permeability transition induced by dinuclear gold(I)–carbene complexes: Potential new antimitochondrial antitumour agents. J. Inorg. Biochem. 2004, 98, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lum, C.T.; Lok, C.-N.; To, W.-P.; Low, K.-H.; Che, C.-M. A Binuclear Gold(I) Complex with Mixed Bridging Diphosphine and Bis(N-Heterocyclic Carbene) Ligands Shows Favorable Thiol Reactivity and Inhibits Tumor Growth and Angiogenesis In Vivo. Angew. Chem. Int. Ed. 2014, 53, 5810–5814. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Bellemin-Laponnaz, S.; Tubaro, C.; Basato, M.; Bogialli, S.; Dolmella, A. Synthesis and biological assays on cancer cells of dinuclear gold complexes with novel functionalised di(N-heterocyclic carbene) ligands. J. Inorg. Biochem. 2014, 141, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.B.; Bernd, M.A.; Schütz, M.; Oberkofler, J.; Pöthig, A.; Reich, R.M.; Kühn, F.E. Synthesis, characterization, and biological studies of multidentate gold(I) and gold(III) NHC complexes. Dalton Trans. 2019, 48, 16615–16625. [Google Scholar] [CrossRef]
- Barnard, P.J.; Wedlock, L.E.; Baker, M.V.; Berners-Price, S.J.; Joyce, D.A.; Skelton, B.W.; Steer, J.H. Luminescence Studies of the Intracellular Distribution of a Dinuclear Gold(I) N-Heterocyclic Carbene Complex. Angew. Chem. Int. Ed. 2006, 45, 5966–5970. [Google Scholar] [CrossRef]
- Wedlock, L.E.; Barnard, P.J.; Filipovska, A.; Skelton, B.W.; Berners-Price, S.J.; Baker, M.V. Dinuclear Au(I) N-heterocyclic carbene complexes derived from unsymmetrical azolium cyclophane salts: Potential probes for live cell imaging applications. Dalton Trans. 2016, 45, 12221–12236. [Google Scholar] [CrossRef] [Green Version]
- Mullick, A.B.; Chang, Y.M.; Ghiviriga, I.; Abboud, K.A.; Tan, W.; Veige, A.S. Human cancerous and healthy cell cytotoxicity studies of a chiral μ-dicarbene–digold(I) metallamacrocycle. Dalton Trans. 2013, 42, 7440. [Google Scholar] [CrossRef]
- Rieb, J.; Dominelli, B.; Mayer, D.; Jandl, C.; Drechsel, J.; Heydenreuter, W.; Sieber, S.A.; Kühn, F.E. Influence of wing-tip substituents and reaction conditions on the structure, properties and cytotoxicity of Ag(I)– and Au(I)–bis(NHC) complexes. Dalton Trans. 2017, 46, 2722–2735. [Google Scholar] [CrossRef]
- Smiataczowa, K.; Kosmalski, J.; Nowacki, A.; Czaja, M.; Warnke, Z. Proton-acceptor properties and capability for mutarotation of some glucosylamines in methanol. Carbohydr. Res. 2004, 339, 1439–1445. [Google Scholar] [CrossRef]
- Anneser, M.R.; Haslinger, S.; Pöthig, A.; Cokoja, M.; Basset, J.-M.; Kühn, F.E. Synthesis and Characterization of an Iron Complex Bearing a Cyclic Tetra-N-heterocyclic Carbene Ligand: An Artificial Heme Analogue? Inorg. Chem. 2015, 54, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Bortolamiol, E.; Rizzolio, F.; Demitri, N.; Visentin, F. Allyl palladium complexes bearing carbohydrate-based N-heterocyclic carbenes: Anticancer agents for selective and potent in vitro cytotoxicity. Appl. Organomet. Chem. 2020, e5876. [Google Scholar] [CrossRef]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef]
- Baron, M.; Battistel, E.; Tubaro, C.; Biffis, A.; Armelao, L.; Rancan, M.; Graiff, C. Single-Step Synthesis of Dinuclear Neutral Gold(I) Complexes with Bridging Di(N-heterocyclic carbene) Ligands and Their Catalytic Performance in Cross Coupling Reactions and Alkyne Hydroamination. Organometallics 2018, 37, 4213–4223. [Google Scholar] [CrossRef]
- Collado, A.; Suárez, A.G.; Martin, A.R.; Slawin, A.M.Z.; Nolan, S.P. Straightforward synthesis of [Au(NHC)X] (NHC = N-heterocyclic carbene, X = Cl, Br, I) complexes. Chem. Commun. 2013, 49, 5541–5543. [Google Scholar] [CrossRef]
- Gil Rubio, J.; Cámara, V.; Bautista, D.; Vicente, J. Dinuclear Alkynyl Gold(I) Complexes Containing Bridging N-Heterocyclic Dicarbene Ligands: New Synthetic Routes and Luminescence. Organometallics 2012, 31, 5414–5426. [Google Scholar] [CrossRef]
- Monticelli, M.; Baron, M.; Tubaro, C.; Bellemin-Laponnaz, S.; Graiff, C.; Bottaro, G.; Armelao, L.; Orian, L. Structural and Luminescent Properties of Homoleptic Silver(I), Gold(I), and Palladium(II) Complexes with nNHC-tzNHC Heteroditopic Carbene Ligands. ACS Omega 2019, 4, 4192–4205. [Google Scholar] [CrossRef]
- Longhi, A.; Baron, M.; Rancan, M.; Bottaro, G.; Armelao, L.; Sgarbossa, P.; Tubaro, C. Possible Synthetic Approaches for Heterobimetallic Complexes by Using nNHC/tzNHC Heteroditopic Carbene Ligands. Molecules 2019, 24, 2305. [Google Scholar] [CrossRef] [Green Version]
- Baron, M.; Tubaro, C.; Biffis, A.; Basato, M.; Graiff, C.; Poater, A.; Cavallo, L.; Armaroli, N.; Accorsi, G. Blue-Emitting Dinuclear N-heterocyclic Dicarbene Gold(I) Complex Featuring a Nearly Unit Quantum Yield. Inorg. Chem. 2012, 51, 1778–1784. [Google Scholar] [CrossRef]
- Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Skelton, B.W.; White, A.H. Dinuclear gold(I) complexes of bridging bidentate carbene ligands: Synthesis, structure and spectroscopic characterisation. Dalton Trans. 2004, 1038–1047. [Google Scholar] [CrossRef]
- Tubaro, C.; Baron, M.; Costante, M.; Basato, M.; Biffis, A.; Gennaro, A.; Isse, A.A.; Graiff, C.; Accorsi, G. Dinuclear gold(I) complexes with propylene bridged N-heterocyclic dicarbene ligands: Synthesis, structures, and trends in reactivities and properties. Dalton Trans. 2013, 42, 10952–10963. [Google Scholar] [CrossRef] [PubMed]
- Hemmert, C.; Poteau, R.; Dominique, F.J.-B.D.; Ceroni, P.; Bergamini, G.; Gornitzka, H. Amide-Functionalized Bis(NHC) Systems: Anion Effect on Gold-Gold Interactions. Eur. J. Inorg. Chem. 2012, 3892–3898. [Google Scholar] [CrossRef]
- Cure, J.; Poteau, R.; Gerber, I.C.; Gornitzka, H.; Hemmert, C. Dimeric Gold Bis(carbene) Complexes by Transmetalation in Water. Organometallics 2012, 31, 619–626. [Google Scholar] [CrossRef]
- Baron, M.; Tubaro, C.; Basato, M.; Isse, A.A.; Gennaro, A.; Cavallo, L.; Graiff, C.; Dolmella, A.; Falivene, L.; Caporaso, L. Insights into the Halogen Oxidative Addition Reaction to Dinuclear Gold(I) Di(NHC) Complexes. Chem. - A Eur. J. 2016, 22, 10211–10224. [Google Scholar] [CrossRef] [PubMed]
- De Frémont, P.; Singh, R.; Stevens, E.D.; Petersen, J.L.; Nolan, S.P. Synthesis, Characterization and Reactivity of N-Heterocyclic Carbene Gold(III) Complexes. Organometallics 2007, 26, 1376–1385. [Google Scholar] [CrossRef]
- Baron, M.; Tubaro, C.; Basato, M.; Biffis, A.; Natile, M.M.; Graiff, C. Dinuclear N-Heterocyclic Dicarbene Gold Complexes in I–III and III–III Oxidation States: Synthesis and Structural Analysis. Organometallics 2011, 30, 4607–4615. [Google Scholar] [CrossRef]
- Zhang, C.; Hemmert, C.; Gornitzka, H.; Cuvillier, O.; Zhang, M.; Sun, R.W. Cationic and Neutral N -Heterocyclic Carbene Gold(I) Complexes: Cytotoxicity, NCI-60 Screening, Cellular Uptake, Inhibition of Mammalian Thioredoxin Reductase, and Reactive Oxygen Species Formation. ChemMedChem 2018, 13, 1218–1229. [Google Scholar] [CrossRef]
- Ott, I. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Barnard, P.J.; Berners-Price, S.J. Targeting the mitochondrial cell death pathway with gold compounds. Co-ord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, J.; Xie, L.; Du, F.; Xu, G.; Xie, Y.; Ling, Q. Synthesis of Chiral Imidazolium Salts from a Carbohydrate and Their Application in Pd-Catalyzed Suzuki–Miyaura Reaction. Catal. Lett. 2014, 144, 1911–1918. [Google Scholar] [CrossRef]
- Salomon, M.F.; Salomon, R.G. The peroxide transfer reaction. J. Am. Chem. Soc. 1979, 101, 4290–4299. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Cell Line | Complex 1 | Complex 2 | Complex 3 | Complex 6 |
|---|---|---|---|---|
| HaCaT | 181 ± 8 | >144 | >85 | 240 ± 15 |
| BALB/c 3T3 | 148 ± 15 | 108 ± 17 | >85 | 241 ± 15 |
| A431 | 162 ± 34 | 137 ± 5 | >85 | 235 ± 16 |
| SVT2 | 139 ± 12 | 118 ± 6 | 72 ± 15 | 207 ± 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tresin, F.; Stoppa, V.; Baron, M.; Biffis, A.; Annunziata, A.; D’Elia, L.; Monti, D.M.; Ruffo, F.; Roverso, M.; Sgarbossa, P.; et al. Synthesis and Biological Studies on Dinuclear Gold(I) Complexes with Di-(N-Heterocyclic Carbene) Ligands Functionalized with Carbohydrates. Molecules 2020, 25, 3850. https://doi.org/10.3390/molecules25173850
Tresin F, Stoppa V, Baron M, Biffis A, Annunziata A, D’Elia L, Monti DM, Ruffo F, Roverso M, Sgarbossa P, et al. Synthesis and Biological Studies on Dinuclear Gold(I) Complexes with Di-(N-Heterocyclic Carbene) Ligands Functionalized with Carbohydrates. Molecules. 2020; 25(17):3850. https://doi.org/10.3390/molecules25173850
Chicago/Turabian StyleTresin, Federica, Valentina Stoppa, Marco Baron, Andrea Biffis, Alfonso Annunziata, Luigi D’Elia, Daria Maria Monti, Francesco Ruffo, Marco Roverso, Paolo Sgarbossa, and et al. 2020. "Synthesis and Biological Studies on Dinuclear Gold(I) Complexes with Di-(N-Heterocyclic Carbene) Ligands Functionalized with Carbohydrates" Molecules 25, no. 17: 3850. https://doi.org/10.3390/molecules25173850
APA StyleTresin, F., Stoppa, V., Baron, M., Biffis, A., Annunziata, A., D’Elia, L., Monti, D. M., Ruffo, F., Roverso, M., Sgarbossa, P., Bogialli, S., & Tubaro, C. (2020). Synthesis and Biological Studies on Dinuclear Gold(I) Complexes with Di-(N-Heterocyclic Carbene) Ligands Functionalized with Carbohydrates. Molecules, 25(17), 3850. https://doi.org/10.3390/molecules25173850