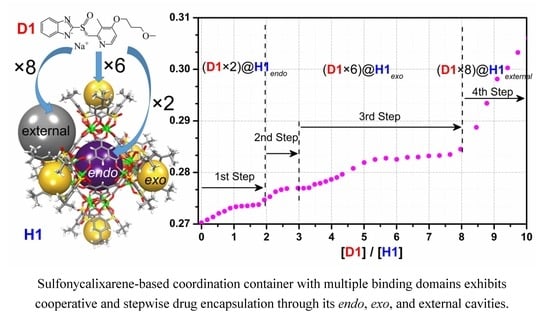
Cooperative Binding and Stepwise Encapsulation of Drug Molecules by Sulfonylcalixarene-Based Metal-Organic Supercontainers
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Solution UV-Vis Titration Experiments
3.3. Calculation of Binding Constants from UV-Vis Titration Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fersht, A. Structure and Mechanism in Protein Science; W. H. Freeman and Company: New York, NY, USA, 1999. [Google Scholar]
- Whitty, A. Cooperativity and biological complexity. Nat. Chem. Biol. 2008, 4, 435–439. [Google Scholar] [CrossRef]
- Williamson, J.R. Cooperativity in macromolecular assembly. Nat. Chem. Biol. 2008, 4, 458–465. [Google Scholar] [CrossRef]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pair Chemistry: Development and Perspectives. Angew. Chem. Int. Ed. 2015, 6400–6441. [Google Scholar] [CrossRef]
- Ercolani, G.; Schiaffino, L. Allosteric, Chelate, and Interannular Cooperativity: A Mise au Point. Angew. Chem. Int. Ed. 2011, 50, 1762–1768. [Google Scholar] [CrossRef]
- Hunter, C.A.; Anderson, H.L. What is Cooperativity? Angew. Chem. Int. Ed. 2009, 48, 7488–7499. [Google Scholar] [CrossRef]
- Cui, Q.; Karplus, M. Allostery and cooperativity revisited. Protein Sci. A Publ. Protein Soc. 2008, 17, 1295–1307. [Google Scholar] [CrossRef] [Green Version]
- Badjic, J.D.; Nelson, A.; Cantrill, S.J.; Turnbull, W.B.; Stoddart, J.F. Multivalency and cooperativity in supramolecular chemistry. Acc. Chem. Res. 2005, 38, 723–732. [Google Scholar] [CrossRef]
- Thordarson, P.; Coumans, R.G.E.; Elemans, J.A.A.W.; Thomassen, P.J.; Visser, J.; Rowan, A.E.; Nolte, R.J.M. Allosterically driven multicomponent assembly. Angew. Chem. Int. Ed. 2004, 43, 4755–4759. [Google Scholar] [CrossRef]
- Mendez-Arroyo, J.; Barroso-Flores, J.; Lifschitz, A.M.; Sarjeant, A.A.; Stern, C.L.; Mirkin, C.A. A multi-state, allosterically-regulated molecular receptor with switchable selectivity. J. Am. Chem. Soc. 2014, 136, 10340–10348. [Google Scholar] [CrossRef]
- Mirtschin, S.; Slabon-Turski, A.; Scopelliti, R.; Velders, A.H.; Severin, K. A Coordination Cage with an Adaptable Cavity Size. J. Am. Chem. Soc. 2010, 132, 14004–14005. [Google Scholar] [CrossRef]
- Ronson, T.K.; Giri, C.; Beyeh, N.K.; Minkkinen, A.; Topic, F.; Holstein, J.J.; Rissanen, K.; Nitschke, J.R. Size-selective encapsulation of hydrophobic guests by self-assembled M4L6 cobalt and nickel cages. Chem. Eur. J. 2013, 19, 3374–3382. [Google Scholar] [CrossRef]
- Therrien, B. Drug Delivery by Water-Soluble Organometallic Cages. Top. Curr. Chem. 2012, 319, 35–55. [Google Scholar]
- Du, S.; Yu, T.-Q.; Liao, W.; Hu, C. Structure modeling, synthesis and X-ray diffraction determination of an extra-large calixarene-based coordination cage and its application in drug delivery. Dalton Trans. 2015, 44, 14394–14402. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. Supramolecular nanoreactors for catalysis. Coord. Chem. Rev. 2016, 324, 106. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Tamura, M.; Fujita, M. Diels-alder in aqueous molecular hosts: Unusual regioselectivity and efficient catalysis. Science 2006, 312, 251–254. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef]
- Dai, F.-R.; Wang, Z. Modular Assembly of Metal–Organic Supercontainers Incorporating Sulfonylcalixarenes. J. Am. Chem. Soc. 2012, 134, 8002–8005. [Google Scholar] [CrossRef]
- Netzer, N.L.; Dai, F.-R.; Wang, Z.; Jiang, C. pH-Modulated Molecular Assemblies and Surface Properties of Metal–Organic Supercontainers at the Air–Water Interface. Angew. Chem. Int. Ed. 2014, 53, 10965–10969. [Google Scholar] [CrossRef]
- Dai, F.-R.; Sambasivam, U.; Hammerstrom, A.J.; Wang, Z. Synthetic Supercontainers Exhibit Distinct Solution versus Solid State Guest-Binding Behavior. J. Am. Chem. Soc. 2014, 136, 7480–7491. [Google Scholar] [CrossRef]
- Dai, F.-R.; Becht, D.C.; Wang, Z. Modulating guest binding in sulfonylcalixarene-based metal-organic supercontainers. Chem. Commun. 2014, 50, 5385–5387. [Google Scholar] [CrossRef]
- Dai, F.-R.; Qiao, Y.; Wang, Z. Designing structurally tunable and functionally versatile synthetic supercontainers. Inorg. Chem. Front. 2016, 3, 243–249. [Google Scholar] [CrossRef]
- Bhuvaneswari, N.; Dai, F.-R.; Chen, Z.N. Sensitive and Specific Guest Recognition through Pyridinium-Modification in Spindle-Like Coordination Containers. Chem. A Eur. J. 2018, 24, 6580–6585. [Google Scholar] [CrossRef]
- Bhuvaneswari, N.; Annamalai, K.P.; Dai, F.-R.; Chen, Z.-N. Pyridinium functionalized coordination containers as highly efficient electrocatalysts for sustainable oxygen evolution. J. Mater. Chem. A 2017, 5, 23559–23565. [Google Scholar] [CrossRef]
- Cheng, L.-J.; Fan, X.-X.; Li, Y.-P.; Wei, Q.-H.; Dai, F.-R.; Chen, Z.-N.; Wang, Z. Engineering solid-state porosity of synthetic supercontainers via modification of exo-cavities. Inorg. Chem. Commun. 2017, 78, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Du, S.; Liao, W. Thiacalixarene-based nanoscale polyhedral coordination cages. Coord. Chem. Rev. 2014, 276, 61–72. [Google Scholar] [CrossRef]
- Liu, M.; Liao, W.; Hu, C.; Du, S.; Zhang, H. Calixarene-Based Nanoscale Coordination Cages. Angew. Chem. Int. Ed. 2012, 51, 1585–1588. [Google Scholar] [CrossRef]
- Xiong, K.; Jiang, F.; Gai, Y.; Yuan, D.; Chen, L.; Wu, M.; Su, K.; Hong, M. Truncated octahedral coordination cage incorporating six tetranuclear-metal building blocks and twelve linear edges. Chem. Sci. 2012, 3, 2321–2325. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Hang, X.; Zhu, X.; Han, H.; Liao, W.; Chen, W. Ultrafine Pt Nanoclusters Confined in a Calixarene-Based {Ni24} Coordination Cage for High-Efficient Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2016, 138, 16236–16239. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kobashi, T.; Shinagawa, R.; Ito, T.; Takaishi, S.; Yamashita, M.; Iki, N. Highly symmetrical tetranuclear cluster complexes supported by p-tert-butylsulfonylcalix[4]arene as a cluster-forming ligand. Eur. J. Inorg. Chem. 2006, 9, 1765–1770. [Google Scholar] [CrossRef]
- Schalley, C.A. Analytical Methods in Supramolecular Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bisswanger, H. Enzyme Kinetics: Principles and Methods, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hill, A.V. The possible effects of the aggregation of the molecules of hæmoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Chenprakhon, P.; Sucharitakul, J.; Panijpan, B.; Chaiyen, P. Measuring Binding Affinity of Protein-Ligand Interaction Using Spectrophotometry: Binding of Neutral Red to Riboflavin-Binding Protein. J. Chem. Educ. 2010, 87, 829–831. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds H1 and H2 are available from the authors. |







| Association Constant (Ka, × 104 M−1) | Hill Coefficient (n) | |
|---|---|---|
| D1 ≅ H1 | 3.58 ± 0.09 | 1.41 ± 0.04 |
| D1 ≅ H1endo | 30.39 ± 2.49 | 1.74 ± 0.18 |
| D1 ≅ H1exo | 9.96 ± 0.16; 5.15 ± 0.08 | 8.00 ± 1.16; 4.03 ± 0.52 |
| D1 ≅ H1external | 0.84 ± 0.04 | 2.46 ± 0.09 |
| D2 ≅ H1 | 6.69 ± 0.11 | 2.30 ± 0.09 |
| D2 ≅ H1endo | 28.08 ± 5.84 | 1.43 ± 0.28 |
| D2 ≅ H1exo | 5.90 ± 0.14 | 3.90 ± 0.41 |
| D2 ≅ H1external | 1.69 ± 0.04 | 3.61 ± 0.59 |
| D1 ≅ H2 | 4.11 ± 0.15 | 1.99 ± 0.09 |
| D2 ≅ H2 | 5.55 ± 0.13 | 1.60 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, T.-P.; Fan, X.-X.; Zheng, G.-Z.; Dai, F.-R.; Chen, Z.-N. Cooperative Binding and Stepwise Encapsulation of Drug Molecules by Sulfonylcalixarene-Based Metal-Organic Supercontainers. Molecules 2020, 25, 2656. https://doi.org/10.3390/molecules25112656
Sheng T-P, Fan X-X, Zheng G-Z, Dai F-R, Chen Z-N. Cooperative Binding and Stepwise Encapsulation of Drug Molecules by Sulfonylcalixarene-Based Metal-Organic Supercontainers. Molecules. 2020; 25(11):2656. https://doi.org/10.3390/molecules25112656
Chicago/Turabian StyleSheng, Tian-Pu, Xin-Xia Fan, Guo-Zong Zheng, Feng-Rong Dai, and Zhong-Ning Chen. 2020. "Cooperative Binding and Stepwise Encapsulation of Drug Molecules by Sulfonylcalixarene-Based Metal-Organic Supercontainers" Molecules 25, no. 11: 2656. https://doi.org/10.3390/molecules25112656
APA StyleSheng, T.-P., Fan, X.-X., Zheng, G.-Z., Dai, F.-R., & Chen, Z.-N. (2020). Cooperative Binding and Stepwise Encapsulation of Drug Molecules by Sulfonylcalixarene-Based Metal-Organic Supercontainers. Molecules, 25(11), 2656. https://doi.org/10.3390/molecules25112656