Enhanced Antigiardial Effect of Omeprazole Analog Benzimidazole Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of Analog Benzimidazoles of Omeprazole
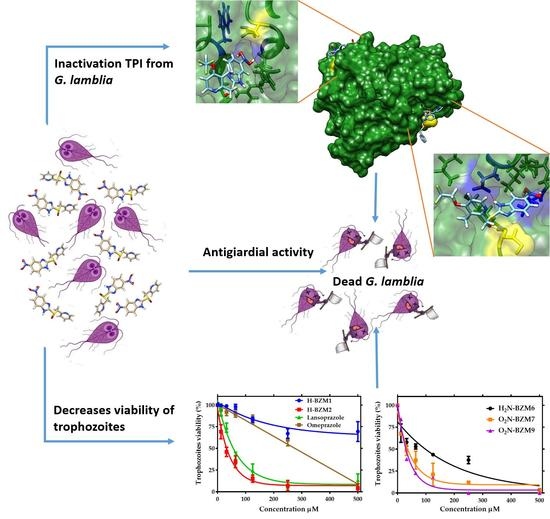
2.2. Dose-Dependent Effect of New Compounds on Giardia lamblia
2.3. Cytotoxic Effect of Compounds on Human Intestinal Caco-2 and HT29 Cells
2.4. In Vitro Screening of Triosephosphate Isomerase Inactivation
2.5. Second-order Inactivation Constant (k2) of TPI Exposed to Selected Hit Compounds
2.6. Structural Studies of TPI Enzyme with the Inhibitory Compound
2.6.1. Circular Dichroism (CD) Assay
2.6.2. Thermal Stability Assays
2.6.3. Intrinsic and Extrinsic Fluorescence Assays
2.7. Molecular Docking Studies
3. Materials and Methods
3.1. Chemicals
3.2. Chemistry
3.3. General Procedure for the Synthesis of Compounds
3.4. In Vitro Assays
3.4.1. Antigiardial Activity
3.4.2. In Vitro Cytotoxicity
3.5. In Vitro Screening of TPI Inactivation
3.5.1. Purification of TPI Recombinant Enzyme
3.5.2. Enzyme Activity Assay
3.5.3. Inactivation of TPI with Compounds
3.6. Spectroscopic Characterization
3.6.1. Circular Dichroism (CD) and Thermal Stability Assays
3.6.2. Intrinsic and Extrinsic Fluorescence Assays
3.7. In Silico Analysis of the TPI Crystallographic Structure from G. lamblia
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berkman, D.S.; Lescano, A.G.; Gilman, R.H.; Lopez, S.L.; Black, M.M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: A follow-up study. Lancet 2002, 359, 564–571. [Google Scholar] [CrossRef]
- Lane, S.; Lloyd, D. Current trends in research into the waterborne parasite giardia. Crit. Rev. Microbiol. 2002, 28, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, J.; Ongerth, J.; Karanis, P. Giardia taxonomy, phylogeny and epidemiology: Facts and open questions. Int. J. Hyg. Environ. Health 2010, 213, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Savioli, L.; Engels, D.; Daumerie, D.; Jannin, J.; Alvar, J.; Asiedu, K.; Gastellu-Etchegorry, M.; Simarro, P.; Mariotti, S.P. Response from savioli and colleagues from the Department of Neglected Tropical Diseases, World Health Organization. PLoS Med. 2006, 3, e283. [Google Scholar] [CrossRef] [Green Version]
- Gardner, T.B.; Hill, D.R. Treatment of giardiasis. Clin. Microbiol. Rev. 2001, 14, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, A.A.; Álvarez, G.; Gonzalez, M.E.; Almirall, P.; Cañete, R.; Cimerman, S.; Ruiz, A.; Pérez, R. The treatment of giardiasis in children: Single-dose tinidazole compared with 3 days of nitazoxanide. Ann. Trop. Med. Parasitol. 2008, 102, 199–207. [Google Scholar] [CrossRef]
- Kulakova, L.; Galkin, A.; Chen, C.Z.; Southall, N.; Marugan, J.J.; Zheng, W.; Herzberg, O. Discovery of novel antigiardiasis drug candidates. Antimicrob. Agents Chemother. 2014, 58, 7303–7311. [Google Scholar] [CrossRef] [Green Version]
- Lemée, V.; Zaharia, I.; Nevez, G.; Rabodonirina, M.; Brasseur, P.; Ballet, J.; Favennec, L. Metronidazole and albendazole susceptibility of 11 clinical isolates of Giardia duodenalis from France. J. Antimicrob. Chemother. 2000, 46, 819–821. [Google Scholar] [CrossRef] [Green Version]
- Upcroft, P.; Upcroft, J.A. Drug targets and mechanisms of resistance in the Anaerobic Protozoa. Clin. Microbiol. Rev. 2001, 14, 150–164. [Google Scholar] [CrossRef] [Green Version]
- Ansell, B.R.; McConville, M.J.; Ma’Ayeh, S.; Dagley, M.J.; Gasser, R.B.; Svärd, S.G.; Jex, A.R. Drug resistance in Giardia duodenalis. Biotechnol. Adv. 2015, 33, 888–901. [Google Scholar] [CrossRef]
- Muller, J.; Hemphill, A.; Müller, N. Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 271–277. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, I.; de la Mora, I.; Marcial-Quino, J.; Gómez-Manzo, S.; Vanoye-Carlo, A.; Navarrete-Vázquez, G.; Colín-Lozano, B.; Gutiérrez-Castrellón, P.; Sierra-Palacios, E.; Navarrete-Vázquez, G.; et al. Proton pump inhibitors drastically modify triosephosphate isomerase from Giardia lamblia at functional and structural levels, providing molecular leads in the design of new antigiardiasic drugs. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 97–107. [Google Scholar] [CrossRef]
- Enriquez-Flores, S.; Rodríguez-Romero, A.; Hernández-Alcántara, G.; Oria-Hernández, J.; Gutiérrez-Castrellón, P.; Pérez-Hernández, G.; de la Mora, I.; Castillo-Villanueva, A.; García-Torres, I.; Méndez, S.-T.; et al. Determining the molecular mechanism of inactivation by chemical modification of triosephosphate isomerase from the human parasite Giardia lamblia: A study for antiparasitic drug design. Proteins Struct. Funct. Bioinform. 2011, 79, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Villanueva, J.; Santos, R.G.; Hernández-Campos, A.; Giulianotti, M.A.; Castillo, R.; Medina-Franco, J.L. Towards a systematic characterization of the antiprotozoal activity landscape of benzimidazole derivatives. Bioorganic Med. Chem. 2010, 18, 7380–7391. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Romo-Mancillas, A.; Hernández-Campos, A.; Yépez-Mulia, L.; Hernández-Luis, F.; Castillo, R. Antiprotozoal activity of proton-pump inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 7351–7354. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin; Yar, M.S.; Mazumder, A. Benzimidazoles: A biologically active compounds. Arab. J. Chem. 2017, 10, S157–S173. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Nava-Zuazo, C.; Castillo-Villanueva, A.; Méndez, S.-T.; Torres-Arroyo, A.; Gómez-Manzo, S.; Marcial-Quino, J.; Ponce-Macotela, M.; Rufino-González, Y.; et al. Novel giardicidal compounds bearing proton pump inhibitor scaffold proceeding through triosephosphate isomerase inactivation. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Navarrete-Vázquez, G.; Cedillo, R.; Hernández-Campos, A.; Yépez, L.; Hernández-Luis, F.; Valdez, J.; Morales, R.; Cortés, R.; Hernández, M.; Castillo, R. Synthesis and antiparasitic activity of 2-(Trifluoromethyl)benzimidazole derivatives. Bioorganic Med. Chem. Lett. 2001, 11, 187–190. [Google Scholar] [CrossRef]
- Valdez, J.; Cedillo, R.; Hernández-Campos, A.; Yépez, L.; Hernández-Luis, F.; Navarrete-Vázquez, G.; Tapia, A.; Cortés, R.; Hernández, M.; Castillo, R. Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorganic Med. Chem. Lett. 2002, 12, 2221–2224. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1H-benzimidazole derivatives. Bioorganic Med. Chem. Lett. 2013, 23, 4221–4224. [Google Scholar] [CrossRef]
- Reyes-Vivas, H.; de la Mora, I.; Castillo-Villanueva, A.; Yépez-Mulia, L.; Hernández-Alcántara, G.; Figueroa-Salazar, R.; García-Torres, I.; Gómez-Manzo, S.; Méndez, S.-T.; Vanoye-Carlo, A.; et al. Giardial triosephosphate isomerase as possible target of the cytotoxic effect of omeprazole in Giardia lamblia. Antimicrob. Agents Chemother. 2014, 58, 7072–7082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Velázquez, G.; Fernández-Lainez, C.; de la Mora, J.I.; de La Portilla, D.C.; Reynoso-Robles, R.; González-Maciel, A.; Ridaura, C.; García-Torres, I.; Gutiérrez-Castrellón, P.; Olivos-García, A.; et al. On the molecular and cellular effects of omeprazole to further support its effectiveness as an antigiardial drug. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constansa, J.F.; Pinol, A.C.; Corominas, J.P. 2-Benzimidazolylalkylthio (or -sulfinyl or -sulfonyl) Derivatives, Their Preparation and Their Application as Medicinal Products. U.S. Patent US4791114A, 13 December 1988. [Google Scholar]
- Raju, M.N.; Kumar, N.U.; Reddy, B.S.; Anitha, N.; Srinivas, G.; Bhattacharya, A.; Mukkanti, K.; Kolla, N.; Bandichhor, R. An efficient synthesis of dexlansoprazole employing asymmetric oxidation strategy. Tetrahedron Lett. 2011, 52, 5464–5466. [Google Scholar] [CrossRef]
- Jang, S.-Y.K.; Tai Won, K.; Sungbum, K.; Byung-Ku, C.; Chang-Ju, K.; Cheol Kyung, H.; Tae Hee, S.; Kwee Hyun, L.; Gwan, S. Process for Preparing (r)-(+)-Lansoprazole and Intermediate Used Therein. Patent Cooperation Treaty (PCT) WO2010068049A2, 17 June 2010. [Google Scholar]
- Liqiang, Y.; Chunzhen, H.; Zhihong, L.; Yuanyuan, H.; Xiangan, M. A Kind of R-Lansoprazole and Preparation Method Thereof and Purposes. CN106946849A, 14 July 2017. [Google Scholar]
- García-Torres, I.; de la Mora, I.; Hernández-Alcántara, G.; Molina-Ortiz, D.; Caballero-Salazar, S.; Olivos-Garcia, A.; Nava, G.; López-Velázquez, G.; Enríquez-Flores, S. First characterization of a microsporidial triosephosphate isomerase and the biochemical mechanisms of its inactivation to propose a new druggable target. Sci. Rep. 2018, 8, 8591. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alcántara, G.; Torres-Larios, A.; Enríquez-Flores, S.; García-Torres, I.; Castillo-Villanueva, A.; Méndez, S.-T.; de la Mora, I.; Gómez-Manzo, S.; Torres-Arroyo, A.; López-Velázquez, G.; et al. Structural and functional perturbation of Giardia lamblia triosephosphate isomerase by modification of a non-catalytic, non-conserved region. PLoS ONE 2013, 8, e69031. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2017, 27, 129–134. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |












| Compounds | IC50 (μM) | CC50 (μM) | IC50 (μM) | |
|---|---|---|---|---|
| Giardia lamblia | Caco-2 (SI) | HT29 (SI) | TPI | |
| H-BZM1 | 676 | 4012 (6) | 3918 (5) | >500 |
| H-BZM2 | 36 | 3184 (88) | 1912 (53) | 37 |
| Lansoprazole | 45 | 2637 (59) | 548 (12) | 66 |
| H2N-BZM6 | 135 | 4006 (29) | 1888 (14) | >500 |
| O2N-BZM7 | 14 | 640 (45) | 519 (37) | 12 |
| O2N-BZM9 | 17 | 663 (39) | 622 (36) | 20 |
| Omeprazole [17] | 300 | - | - | 225 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ochoa, B.; Gómez-Manzo, S.; Sánchez-Carrillo, A.; Marcial-Quino, J.; Rocha-Ramírez, L.M.; Santos-Segura, A.; Ramírez-Nava, E.J.; Arreguin-Espinosa, R.; Cuevas-Cruz, M.; Méndez-Tenorio, A.; et al. Enhanced Antigiardial Effect of Omeprazole Analog Benzimidazole Compounds. Molecules 2020, 25, 3979. https://doi.org/10.3390/molecules25173979
Hernández-Ochoa B, Gómez-Manzo S, Sánchez-Carrillo A, Marcial-Quino J, Rocha-Ramírez LM, Santos-Segura A, Ramírez-Nava EJ, Arreguin-Espinosa R, Cuevas-Cruz M, Méndez-Tenorio A, et al. Enhanced Antigiardial Effect of Omeprazole Analog Benzimidazole Compounds. Molecules. 2020; 25(17):3979. https://doi.org/10.3390/molecules25173979
Chicago/Turabian StyleHernández-Ochoa, Beatriz, Saúl Gómez-Manzo, Adrián Sánchez-Carrillo, Jaime Marcial-Quino, Luz María Rocha-Ramírez, Araceli Santos-Segura, Edson Jiovany Ramírez-Nava, Roberto Arreguin-Espinosa, Miguel Cuevas-Cruz, Alfonso Méndez-Tenorio, and et al. 2020. "Enhanced Antigiardial Effect of Omeprazole Analog Benzimidazole Compounds" Molecules 25, no. 17: 3979. https://doi.org/10.3390/molecules25173979
APA StyleHernández-Ochoa, B., Gómez-Manzo, S., Sánchez-Carrillo, A., Marcial-Quino, J., Rocha-Ramírez, L. M., Santos-Segura, A., Ramírez-Nava, E. J., Arreguin-Espinosa, R., Cuevas-Cruz, M., Méndez-Tenorio, A., & Calderón-Jaimes, E. (2020). Enhanced Antigiardial Effect of Omeprazole Analog Benzimidazole Compounds. Molecules, 25(17), 3979. https://doi.org/10.3390/molecules25173979