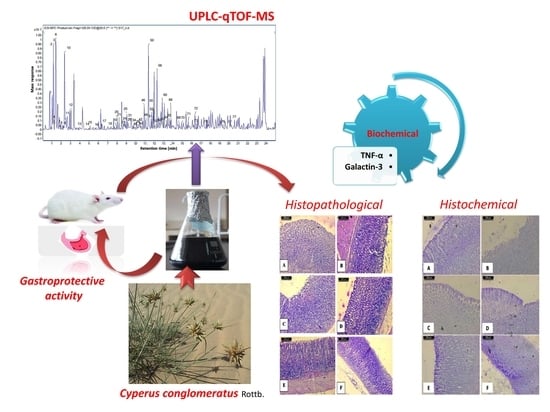
UPLC-qTOF-MS Phytochemical Profile and Antiulcer Potential of Cyperus conglomeratus Rottb. Alcoholic Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of C. Conglomeratus Alcohol Extract Using UPLC-Q to f-MS
2.1.1. Organic Acids
2.1.2. Hydroxybenzoic Acids
2.1.3. Hydroxycinnamic Acids
2.1.4. Cyperaquinones
2.1.5. Flavonoids
2.1.6. Coumestans/Stilbenes
2.1.7. Aurones
2.1.8. Procyanidins
2.2. Gastroprotective Activity
2.3. Biochemical Results
2.4. Histopathological Results
2.5. Histochemical Results
3. Materials and Methods
3.1. Plant Material Collection and Preparation of Extract
3.2. High-Resolution Ultra-Performance Liquid Chromatography-Mass Spectrometry Analysis (UPLC-ESI–Qtof-MS)
3.3. Experimental Animals
3.4. Ulcer Induction
3.5. Experimental Design and Animal Grouping
3.6. Galactin-3 and TNF- α Determination
3.7. Histopathology
3.8. Gastric Mucosal Glycoprotein Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asnaashari, S.; Dastmalchi, S.; Javadzadeh, Y. Gastroprotective effects of herbal medicines (roots). Int. J. Food Prop. 2018, 21, 902–920. [Google Scholar] [CrossRef] [Green Version]
- Park, J.U.; Kang, J.H.; Rahman, M.A.A.; Hussain, A.; Cho, J.S.; Lee, Y.I. Gastroprotective effects of plants extracts on gastric mucosal injury in experimental sprague-dawley rats. BioMed Res. Int. 2019, 2019, 8759708. [Google Scholar] [CrossRef] [Green Version]
- Bansal, V.K.; Goel, R.K. Gastroprotective effect of Acacia nilotica young seedless pod extract: Role of polyphenolic constituents. Asian Pac. J. Trop. Med. 2012, 5, 523–528. [Google Scholar] [CrossRef]
- de Lira Mota, K.S.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, Â.; Monteiro Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrag, A.R.H.; Abdallah, H.M.I.; Khattab, A.R.; Elshamy, A.I.; Gendy, A.; Mohamed, T.A.; Farag, M.A.; Efferth, T.; Hegazy, M.F. Antiulcer activity of Cyperus alternifolius in relation to its UPLC-MS metabolite fingerprint: A mechanistic study. Phytomedicine 2019, 62, 152970. [Google Scholar] [CrossRef]
- Elshamy, A.I.; El-Shazly, M.; Yassine, Y.M.; El-Bana, M.A.; Farrag, A.-R.; Nassar, M.I.; Singab, A.N.; Noji, M.; Umeyama, A. Phenolic constituents, anti-inflammatory and antidiabetic activities of Cyperus laevigatus L. Pharmacogn. J. 2017, 9, 828–833. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Mishra, S. Hepatoprotective activity of rhizomes of Cyperus rotundus Linn against carbon tetrachloride-induced hepatotoxicity. Indian J. Pharm. Sci. 2005, 67, 84–88. [Google Scholar]
- Thebtaranonth, C.; Thebtaranonth, Y.; Wanauppathamkul, S.; Yuthavong, Y. Antimalarial sesquiterpenes from tubers of Cyperus rotundus: Structure of 10, 12-peroxycalamenene, a sesquiterpene endoperoxide. Phytochemistry 1995, 40, 125–128. [Google Scholar] [CrossRef]
- Kumar, K.H.; Razack, S.; Nallamuthu, I.; Khanum, F. Phytochemical analysis and biological properties of Cyperus rotundus L. Ind. Crops Prod. 2014, 52, 815–826. [Google Scholar] [CrossRef]
- Raut, N.A.; Gaikwad, N.J. Antidiabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats. Fitoterapia 2006, 77, 585–588. [Google Scholar] [CrossRef]
- Nassar, M.I.; Yassine, Y.M.; Elshamy, A.I.; El-Beih, A.A.; El-Shazly, M.; Singab, A.N.B. Essential oil and antimicrobial activity of aerial parts of Cyperus leavigatus L. (Family: Cyperaceae). J. Essent. Oil Bear. Plant. 2015, 18, 416–422. [Google Scholar] [CrossRef]
- Nassar, M.I.; Abdel-Razik, A.F.; El-Khrisy, E.E.-D.A.; Dawidar, A.-A.M.; Bystrom, A.; Mabry, T.J. A benzoquinone and flavonoids from Cyperus alopecuroides. Phytochemistry 2002, 60, 385–387. [Google Scholar] [CrossRef]
- Abdel-Razik, A.F.; Nassar, M.I.; El-Khrisy, E.-D.A.; Dawidar, A.-A.M.; Mabry, T.J. New prenylflavans from Cyperus conglomeratus. Fitoterapia 2005, 76, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Seabra, R.M.; Moreira, M.M.; Costa, M.A.C.; Paul, M.I. 6,3′,4′-trihydroxy-4-methoxy-5-methylaurone from Cyperus capitatus. Phytochemistry 1995, 40, 1579–1580. [Google Scholar] [CrossRef]
- Seabra, R.M.; Silva, A.M.S.; Andrade, P.B.; Manuela Moreira, M. Methylaurones from Cyperus capitatus. Phytochemistry 1998, 48, 1429–1432. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.-W.; Yu, C.-Y.; Lu, Y.; Chang, Y.; Zou, Z.-M. Norcyperone, a novel skeleton norsesquiterpene from Cyperus rotundus L. Molecules 2008, 13, 2474–2481. [Google Scholar] [CrossRef]
- Gamal, M.; Hani, K.M.; Sameh, E.S.; Sabrin, I.R. A review: Compounds isolated from Cyperus species (Part I): Phenolics and nitrogenous. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 51–67. [Google Scholar]
- Hisham, A.; Rameshkumar, K.B.; Sherwani, N.; Al-Saidi, S.; Al-Kindy, S. The composition and antimicrobial activities of Cyperus conglomeratus, Desmos chinensis var. lawii and Cyathocalyx zeylanicus essential oils. Nat. Prod. Commun. 2012, 7, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Al-Hazmi, G.H.; Awaad, A.S.; Alothman, M.R.; Alqasoumi, S.I. Anticandidal activity of the extract and compounds isolated from Cyperus conglomertus Rottb. Saudi Pharm. J. 2018, 26, 891–895. [Google Scholar] [CrossRef]
- Konturek, P.C.; Duda, A.; Brzozowski, T.; Konturek, S.; Kwiecien, S.; Drozdowicz, D.; Pajdo, R.; Meixner, H.; Hahn, E. Activation of genes for superoxide dismutase, interleukin-1ß, tumor necrosis factor-a, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand. J. Gastroenterol. 2000, 35, 452–463. [Google Scholar]
- Kwiecien, S.; Brzozowski, T.; Konturek, S. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar] [PubMed]
- Elshamy, A.I.; Farrag, A.H.; Mohamed, S.H.; Ali, N.A.; Mohamed, T.A.; Menshawy, M.; Zaglool, A.; Efferth, T.; Hegazy, M.-E. Gastroprotective effects of ursolic acid isolated from Ochrosia elliptica on ethanol-induced gastric ulcer in rats. Med. Chem. Res. 2020, 29, 113–125. [Google Scholar] [CrossRef]
- Elkady, W.M.; Ayoub, I.M.; Abdel-Mottaleb, Y.; ElShafie, M.F.; Wink, M. Euryops pectinatus L. Flower extract inhibits p-glycoprotein and reverses multi-drug resistance in cancer cells: A mechanistic study. Molecules 2020, 25, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef]
- Faheem, S.A.; Saeed, N.M.; El-Naga, R.N.; Ayoub, I.M.; Azab, S.S. Hepatoprotective effect of cranberry nutraceutical extract in non-alcoholic fatty liver model in rats: Impact on insulin resistance and Nrf-2 expression. Front. Pharmacol. 2020, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.-Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Nie, B.; Liu, T.; Yuan, F.; Feng, F.; Zhang, Y.; Zhou, W.; Xu, X.; Yao, M.; Zhang, F. Simultaneous determination of coumarin and its derivatives in tobacco products by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2016, 21, 1511. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Sharaf Eldin, M.G.; Kassem, H.; Abou el Fetouh, M. Metabolome classification of Brassica napus L. organs via UPLC–QTOF–PDA–MS and their anti-oxidant potential. Phytochem. Anal. 2013, 24, 277–287. [Google Scholar] [CrossRef]
- Thomson, R. Naturally Occurring Quinones; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Yang, M.; Wang, W.; Sun, J.; Zhao, Y.; Liu, Y.; Liang, H.; Guo, D.A. Characterization of phenolic compounds in the crude extract of Hedysarum multijugum by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3833–3841. [Google Scholar] [CrossRef]
- Morikawa, T.; Xu, F.; Matsuda, H.; Yoshikawa, M. Structures of novel norstilbene dimer, longusone A, and three new stilbene dimers, longusols A, B, and C, with antiallergic and radical scavenging activities from Egyptian natural medicine Cyperus longus. Chem. Pharm. Bull. 2010, 58, 1379–1385. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Khattab, A.R.; Maamoun, A.A.; Kropf, M.; Heiss, A.G. UPLC-MS metabolome based classification of Lupinus and Lens seeds: A prospect for phyto-equivalency of its different accessions. Food Res. Int. 2019, 115, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, Y.; Ye, Q.; Liang, Y.; He, X.; Zhou, Z.; Feng, Z. A new isoflavonoid from the rhizomes of Cyperus rotundus. Asian J. Chem. 2014, 26, 3967–3970. [Google Scholar] [CrossRef]
- Abdel-Mogib, M.; Basaif, S.; Ezmirly, S. Two novel flavans from Cyperus conglomeratus. Pharmazie 2000, 55, 693–695. [Google Scholar] [PubMed]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G. Anti-acetylcholinesterase potential and metabolome classification of 4 Ocimum species as determined via UPLC/qTOF/MS and chemometric tools. J. Pharm. Biomed. Anal. 2016, 125, 292–302. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Contreras, M.M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef]
- Rabelo, A.S.; Serafini, M.R.; Rabelo, T.K.; de Melo, M.G.D.; da Silva Prado, D.; Gelain, D.P.; Moreira, J.C.F.; dos Santos Bezerra, M.; da Silva, T.B.; Costa, E.V.; et al. Chemical composition, antinociceptive, anti-inflammatory and redox properties in vitro of the essential oil from Remirea maritima Aubl. (Cyperaceae). BMC Complementary Altern. Med. 2014, 14, 514. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Porzel, A.; Schmidt, J.; Wessjohann, L.A. Metabolite profiling and fingerprinting of commercial cultivars of Humulus lupulus L. (hop): A comparison of MS and NMR methods in metabolomics. Metabolomics 2012, 8, 492–507. [Google Scholar] [CrossRef]
- Shen, J.; Wei, J.; Li, L.; Ouyang, H.; Chang, Y.; Chen, X.; He, J. “Development of a HPLC-MS/MS method to determine 11 bioactive compounds in Tongmai Yangxin Pill and application to a pharmacokinetic study in rats. Evid. Based Compl. Alt. 2018, 2018, 6460393. [Google Scholar] [CrossRef]
- Amesty, Á.; Burgueño-Tapia, E.; Joseph-Nathan, P.; Ravelo, Á.G.; Estévez-Braun, A. Benzodihydrofurans from Cyperus teneriffae. J. Nat. Prod. 2011, 74, 1061–1065. [Google Scholar] [CrossRef]
- Guo, J.; Liu, D.; Nikolic, D.; Zhu, D.; Pezzuto, J.M.; van Breemen, R.B. In vitro metabolism of isoliquiritigenin by human liver microsomes. Drug Metab. Dispos. 2008, 36, 461. [Google Scholar] [CrossRef] [Green Version]
- Alanís, R.M.; Kennedy, J.F. Dictionary of Food Compounds with CD-Rom: Additives. In Flavors, and Ingredients; Yannai, S., Ed.; CRC: Washington, DC, USA, 2004. [Google Scholar]
- Appleyard, C.; McCafferty, D.; Tigley, A.; Swain, M.; Wallace, J. Tumor necrosis factor mediation of NSAID-induced gastric damage: Role of leukocyte adherence. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 270, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Santucci, L.; Fiorucci, S.; Di Matteo, F.M.; Morelli, A. Role of tumor necrosis factor α release and leukocyte margination in indomethacin-induced gastric injury in rats. Gastroenterology 1995, 108, 393–401. [Google Scholar] [CrossRef]
- Wallace, J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.; Peng, J.; Zhang, Z.; Jiang, D.-J.; Li, Y.-J. Role of endogenous nitric oxide synthase inhibitor in gastric mucosal injury. Can. J. Physiol. Pharm. 2008, 86, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Freitas, R.; Athayde, M.; Boligon, A.; Augusti, P.; Somacal, S.; Rocha, M.; Bauermann, L. Effect of Vernonia cognata on oxidative damage induced by ethanol in rats. Hum. Exp. Toxicol. 2011, 30, 675–684. [Google Scholar] [CrossRef]
- Ganguly, K.; Maity, P.; Reiter, R.J.; Swarnakar, S. Effect of melatonin on secreted and induced matrix metalloproteinase-9 and-2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 2005, 39, 307–315. [Google Scholar] [CrossRef]
- Amagase, K.; Yokota, M.; Tsukimi, Y.; Okabe, S. Characterization of “unhealed gastric ulcers” produced with chronic exposure of acetic acid ulcers to indomethacin in rats. J. Physiol. Pharmacol. 2003, 54, 349–360. [Google Scholar]
- Potrich, F.B.; Allemand, A.; da Silva, L.M.; dos Santos, A.C.; Baggio, C.H.; Freitas, C.S.; Mendes, D.A.G.B.; Andre, E.; de Paula Werner, M.F.; Marques, M.C.A. Antiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L.: Involvement of the antioxidant system. J. Ethnopharmacol. 2010, 130, 85–92. [Google Scholar] [CrossRef]
- Tsukimi, Y.; Okabe, S. Recent advances in gastrointestinal pathophysiology: Role of heat shock proteins in mucosal defense and ulcer healing. Biol. Pharm. Bull. 2001, 24, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Salga, M.S.; Ali, H.M.; Abdulla, M.A.; Abdelwahab, S.I. Gastroprotective activity and mechanism of novel dichlorido-zinc (II)-4-(2-(5-methoxybenzylideneamino) ethyl) piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chem. Biol. Interact. 2012, 195, 144–153. [Google Scholar] [CrossRef]
- Choi, J.-i.; Raghavendran, H.R.B.; Sung, N.-Y.; Kim, J.-H.; Chun, B.S.; Ahn, D.H.; Choi, H.-S.; Kang, K.-W.; Lee, J.-W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.; Pesek, I. Glomerular tumor necrosis factor and interleukin 1 during acute aminonucleoside nephrosis. An immunohistochemical study. Lab. Investig. 1991, 64, 21–28. [Google Scholar] [PubMed]
- Faubion, W.A.; Gores, G.J. Death receptors in liver biology and pathobiology. Hepatology 1999, 29, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Adhikary, B.; Chand, S.; Maity, B.; Bandyopadhyay, S.K.; Chattopadhyay, S. Molecular mechanism of indomethacin-induced gastropathy. Free Radic. Biol. Med. 2012, 52, 1175–1187. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The role of galectins as modulators of metabolism and inflammation. Mediat. Inflamm. 2018, 2018, 9186940. [Google Scholar] [CrossRef] [Green Version]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, 208884. [Google Scholar] [CrossRef] [Green Version]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, D.D.; Wakefield, T.W.; Diaz, J.A. The role of galectin-3 and galectin-3–binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef] [Green Version]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Sidahmed, H.M.A.; Azizan, A.H.S.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Taha, M.M.E.; Hadi, A.H.A.; Ketuly, K.A.; Hashim, N.M.; Loke, M.F. Gastroprotective effect of desmosdumotin C isolated from Mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: Possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-Helicobacter pylori activity. BMC Complem. Altern. Med. 2013, 13, 183. [Google Scholar]
- Park, S.W.; Oh, T.Y.; Kim, Y.S.; Sim, H.; Park, S.J.; Jang, E.J.; Park, J.S.; Baik, H.W.; Hahm, K.B. Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. J. Gastroenterol. Hepatol. 2008, 23, 976–984. [Google Scholar] [CrossRef]
- Silva, M.I.; Moura, M.A.; de Aquino Neto, M.R.; da Rocha, T.A.; Rocha, N.F.; de Carvalho, A.M.; Macêdo, D.S.; Vasconcelos, S.M.; de Sousa, D.P.; Viana, G.S.; et al. Gastroprotective activity of isopulegol on experimentally induced gastric lesions in mice: Investigation of possible mechanisms of action. N-S. Arch. Pharmacol. 2009, 380, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sannomiya, M.; Fonseca, V.B.; Da Silva, M.; Rocha, L.; Dos Santos, L.; Hiruma-Lima, C.; Brito, A.S.; Vilegas, W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J. Ethnopharmacol. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Algebali, M.; Menze, E.T.; Ayoub, I.M.; Tadros, M.; Esmat, A. Macro and microscopic gastroprotective effects of grape seed extract on the gastric ulcer experimentally induced by alcohol. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 113–123. [Google Scholar] [CrossRef]
- Bhoumik, D.; Masresha, B.; Mallik, A. Antiulcer properties of herbal drugs: A review. Int. J. Biomed. Res. 2017, 8, 116–124. [Google Scholar]
- Golbabapour, S.; Gwaram, N.S.; Hassandarvish, P.; Hajrezaie, M.; Kamalidehghan, B.; Abdulla, M.A.; Ali, H.M.; Hadi, A.H.A.; Majid, N.A. Gastroprotection studies of Schiff base zinc (II) derivative complex against acute superficial hemorrhagic mucosal lesions in rats. PLoS ONE 2013, 8, 75036. [Google Scholar] [CrossRef] [Green Version]
- Hajrezaie, M.; Golbabapour, S.; Hassandarvish, P.; Gwaram, N.S.; Hadi, A.H.A.; Ali, H.M.; Majid, N.; Abdulla, M.A. Acute toxicity and gastroprotection studies of a new schiff base derived copper (II) complex against ethanol-induced acute gastric lesions in rats. PLoS ONE 2012, 7, 51537. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, I.; Agrawal, S.; Goel, R.K. Status of inflammatory markers and growth factor in gastric ulcer protective effects of Punica granatum L. peel extract in rat. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 12–17. [Google Scholar] [CrossRef]
- Bauer, H.; Jung, T.; Tsikas, D.; Stichtenoth, D.; FRÖLICH, C.; Neumann, C. Nitric oxide inhibits the secretion of T-helper 1-and T-helper 2-associated cytokines in activated human T cells ‘pa. Immunology 1997, 90, 205–211. [Google Scholar] [CrossRef]
- Batista, L.; De Morais, L.G.; De Almeida, A.; Magri, L.; Calvo, T.; Ferreira, A.; Pellizzon, C.; Hiruma-Lima, C.; Vilegas, W.; Sano, P.; et al. Ulcer healing and mechanism(s) of action involved in the gastroprotective activity of fractions obtained from Syngonanthus arthrotrichus and Syngonanthus bisulcatus. BMC Complementary Altern. Med. 2015, 15, 391. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.A.; Balan, T.; Suppaiah, V.; Ahmad, S.; Jamaludin, F. Mechanism+(s) of action involved in the gastroprotective activity of Muntingia calabura. J. Ethnopharmacol. 2014, 151, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelwahab, S.I.; Mohan, S.; Abdulla, M.A.; Sukari, M.A.; Abdul, A.B.; Taha, M.M.E.; Syam, S.; Ahmad, S.; Lee, K.-H. The methanolic extract of Boesenbergia rotunda (L.) Mansf. and its major compound pinostrobin induces anti-ulcerogenic property in vivo: Possible involvement of indirect antioxidant action. J. Ethnopharmacol. 2011, 137, 963–970. [Google Scholar] [CrossRef] [PubMed]
- La Casa, C.; Villegas, I.; De La Lastra, C.A.; Motilva, V.; Calero, M.M. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Martín, M.; De La Lastra, C.A.; Motilva, V.; La Casa, C. Antiulcer and gastroprotective activity of flavonic compounds: Mechanisms involved. Stud. Nat. Prod. Chem. 2000, 22, 419–456. [Google Scholar]
- Sumbul, S.; Ahmad, M.A.; Mohd, A.; Mohd, A. Role of phenolic compounds in peptic ulcer: An overview. J. Pharm. Bioallied Sci. 2011, 3, 361–367. [Google Scholar] [PubMed]
- Trautmann, M.; Peskar, B.M.; Peskar, B.A. Aspirin-like drugs, ethanol-induced rat gastric injury and mueosal eicosanoid release. Eur. J. Pharmacol. 1991, 201, 53–58. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.-H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef]
- Abdel Motaal, A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- Iqbal, M.; Verpoorte, R.; Korthout, H.A.; Mustafa, N.R. Phytochemicals as a potential source for TNF-α inhibitors. Phytochem. Rev. 2013, 12, 65–93. [Google Scholar] [CrossRef]
- Maamoun, A.A.; El-akkad, R.H.; Farag, M.A. Mapping metabolome changes in Luffa aegyptiaca Mill fruits at different maturation stages via MS-based metabolomics and chemometrics. J. Adv. Res. 2019. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: New York, NY, USA, 2008. [Google Scholar]
- Nordin, N.; Salama, S.M.; Golbabapour, S.; Hajrezaie, M.; Hassandarvish, P.; Kamalidehghan, B.; Majid, N.A.; Hashim, N.M.; Omar, H.; Fadaienasab, M. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (King) Heusden against ethanol-induced acute gastric lesion in animal models. PLoS ONE 2014, 9, 111925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |




| No | Name | Formula | RT | [M − H] m/z | Exact Mass | Diff (ppm) | Ms Fragmentation | Class | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Quinic acid | C7H12O6 | 1.038 | 191.0584 | 192.0657 | −9.16 | 131, 127, 85 | Organic acid | [5] |
| 2. | Malic acid | C4H6O5 | 1.129 | 133.0162 | 134.0235 | 0.05 | 115 | Organic acid | [23] |
| 3. | Unidentified | C30H30O9 | 1.147 | 533.1781 | 7.33 | 473, 377, 191, 133 | |||
| 4. | Tetrahydroxypentanoic acid | C5H10O6 | 1.312 | 165.0423 | 166.0495 | −10.85 | 75 | Organic acid | |
| 5. | Unidentified | - | 1.404 | 195.8125 | 162.84, 61.98 | ||||
| 6. | Citric acid/isocitric acid | C6H8O7 | 1.449 | 191.0222 | 192.0295 | −12.96 | 159, 111, 87, 67 | Organic acid | [5] |
| 7. | Malic acid | C4H6O5 | 2.089 | 133.0158 | 134.0232 | −11.99 | 115 | Organic acid | [23] |
| 8. | Fumaric acid | C4H4O4 | 2.226 | 115.0052 | 116.0124 | −12.83 | 99, 87,72, 71 | Organic acid | |
| 9. | Unidentified | C23H10O2 | 2.272 | 317.0588 | 318.0654 | 8.42 | 225, 197, 150 | ||
| 10. | Unidentified | - | 2.363 | 195.813 | - | 162.84, 80.97 | |||
| 11. | Leucine-hexose | C12H23 NO7 | 2.683 | 292.1419 | −6.02 | 257, 130, 84 | Amino acid | ||
| 12. | Homocitric acid | C7H10O7 | 3.094 | 205.0378 | 206.0449 | −10.92 | 179, 161, 126, 111, 85 | Organic acid | |
| 13. | Homocitric acid (Isomer) | C7H10O7 | 4.054 | 205.0376 | 206.0449 | −10.92 | 161, 126, 111, 87 | Organic acid | |
| 14. | Homocitric acid (Isomer) | C7H10O7 | 5.014 | 205.037 | 206.0439 | −6.06 | 161, 126, 111, 87 | Organic acid | |
| 15. | Dihydroxybenzoic acid | C7H6O4 | 5.06 | 153.0213 | 154.0283 | −11.03 | 109 | Phenolic acid | [5] |
| 16. | Dihydroxybenzoic acid methyl ester | C8H8O4 | 5.745 | 167.0363 | 168.0435 | −7.4 | 151, 125, 123, 108, 81 | Phenolic acid derivative | |
| 17. | Dihydroxy benzoic acid-O-hexoside | C13H16O9 | 6.339 | 315.0751 | 316.0821 | −8.37 | 153, 97 | Phenolic acid derivative | |
| 18. | Hexahydroxyflavan (gallocatechin) | C15H14O7 | 7.527 | 305.0702 | 306.0771 | −10.45 | 179, 167, 125 | Flavanol | |
| 19. | Dihydroxy benzoic acid isomer | C7H6O4 | 7.938 | 153.0214 | 154.0286 | −13.03 | 109 | Phenolic acid | [5] |
| 20. | Unidentified | C11H20O8 | 8.075 | 279.1104 | 280.1174 | −5.74 | 117, 89 | ||
| 21. | Dihydroxy benzoic acid methyl ester hexoside | C14H18O9 | 8.167 | 329.09 | 330.0972 | −6.35 | 270 [M – H − COOCH3]−, 167 [M – H − 162]−, 153, 125, 108 | Phenolic glycoside | [24] |
| 22. | O-hexosyl-O-methyl-myo-inositol-dihydroxy benzoic acid | C20H30O14 | 8.258 | 493.1601 | 494.166 | −4.85 | 331 [M − H − 162]−, 293, 243, 209, 167, 137 [M − H − 162 − 194]−, 89 | Phenolic glycoside | |
| 23. | Salicylic acid | C7H6O3 | 8.624 | 137.0257 | 138.0329 | −8.64 | 93 [–COO]− | Phenolic acid | |
| 24. | Piscidic Acid (p-hydroxybenzoyl) tartaric acid | C11H12O7 | 8.944 | 255.0529 | 256.0613 | −11.6 | 165, 149, 135 | Organic acid | |
| 25. | Benzoyl tartaric acid | C11H10O7 | 9.035 | 253.0378 | 254.0449 | −8.69 | 195, 123 | Organic acid | |
| 26. | Procyanidin B dimer | C30H26O12 | 9.172 | 577.1401 | 578.148 | −9.52 | 451, 425, 407, 289 | Proanthocyanidin | [25,26] |
| 27. | Hexahydroxyflavan | C15H14O7 | 9.218 | 305.0699 | 306.0767 | −8.93 | 179, 167, 125 | Flavanol | |
| 28. | C-hexosylprocyanidin B dimer | C36H36O17 | 9.263 | 739.1929 | 740.2011 | −7.92 | 649 [M − H − 90]−, 619 [M − H − 120]−, 587 [M − H − 152]− RDA, 449 [M − H − 289]− QM, 289 | Proanthocyanidin | |
| 29. | Unidentified | C17H22O11 | 9.309 | 401.1124 | 402.1163 | −0.14 | 313, 267, 193, 151 | ||
| 30. | (epi)catechin | C15H14O6 | 9.355 | 289.0742 | 290.0813 | −7.67 | 245, 205, 203, 179 | Flavanol | |
| 31. | Dimethoxyhomophthalic acid | C11H12O6 | 9.492 | 239.0587 | 240.0655 | −8.85 | 195 [M − H − COO]−, 179 [M − H − 2 OCH3]−, 149 [M − H − 2 COOH]−, 133, 107, 87 | Organic acid | |
| 32. | Caffeic acid | C9H8O4 | 9.583 | 179.0366 | 180.0439 | −9.09 | 135 | Phenolic acid | |
| 33. | Hydroxymethoxycinnamaldehyde | C10H10O3 | 9.629 | 177.0574 | 178.0647 | −9.42 | 134 | Aldehyde | |
| 34. | O-Caffeoylquinic acid | C16H18O9 | 9.72 | 353.0915 | 354.0977 | −7.38 | 191 | Phenolic acid | |
| 35. | O-Syringoylquinic acid | C16H20O10 | 9.903 | 371.1001 | 372.106 | −0.86 | 121 | Phenolic acid | |
| 36. | Caffeoquinone | C9H6O4 | 10.04 | 177.0213 | 178.0287 | −11.65 | 149 [M − H − CO]−, 135, 133 [M − H − CO2]−,105 [M − H − COOCH2]−, 93 | Phenolic acid | [27] |
| 37. | Unknown | C14H24O10 | 10.086 | 351.1332 | 352.1398 | −8.01 | 293, 249, 191, 173, 133 (Malic acid) | Organic acid | |
| 38. | Procyanidin B dimer | C30H26O12 | 10.177 | 577.1408 | 578.1483 | 0.08 | 451 [M − H − 126]−, 425 [M − H − 152]−, 407 [M − H − 152 − 18]−, 289 [M − H − 288]− | Proanthocyanidin | [25,26] |
| 39. | Syringoylmalic acid | C13H14O9 | 10.223 | 313.06 | 314.0669 | −10.03 | 197, 153, 121 | Phenolic acid | |
| 40. | Syringic acid | C9H10O5 | 10.269 | 197.0476 | 198.0547 | −9.35 | 167 [M − H − 2CH3]−, 121, 78 | Phenolic acid | |
| 41. | Dihydroxyhomophthalic acid dimethyl ester | C11H12O6 | 10.451 | 239.0586 | 240.0657 | −9.48 | 195 [M − H − COO]−, 179 [M − H − 2 OCH3]−, 149 [M − H − 2 COOH]−, 133, 107, 87 | Organic acid | |
| 42. | Hydroxycinnamic acid | C9H8O3 | 10.497 | 163.0421 | 164.0496 | −13.56 | 119 | Phenolic acid | [5] |
| 43. | (epi)-Catechin | C15H14O6 | 10.634 | 289.0738 | 290.0808 | −6.07 | 245, 205, 203, 179 | Flavanol | |
| 44. | Eriodictyol | C15H12O6 | 10.726 | 287.0587 | 288.0661 | −9.31 | 151, 135 | Flavanone | |
| 45. | Scopoletin | C10H8O4 | 10.817 | 191.0349 | 192.0422 | 0.5 | 176, 148, 134,107 | Coumarin | [17] |
| 46. | Hydroxydimethoxycinnamic acid | C11H12O5 | 10.908 | 223.0635 | 224.0708 | −10.52 | 163 [M − H − 2 OCH3]−, 133, 117, 91 | Phenolic acid | [28] |
| 47. | Ferulic acid | C10H10O4 | 10.954 | 193.0526 | 194.0599 | −10.33 | 149,134, 107 | Phenolic acid | |
| 48. | Dihydrocyperaquinone | C14H12O4 | 11.091 | 243.0679 | 244.075 | −5.81 | 201 [M − H − 42]−, 159 | [29] | |
| 49. | Caffeoquinone isomer | C9H6O4 | 11.274 | 177.0205 | 178.0279 | −7.02 | 133, 89 | Phenolic acid | |
| 50. | Trihydroxycoumestan | C15H8O6 | 11.365 | 283.0275 | 284.035 | −10.13 | 255 [M − H − CO]−, 239 [M − H − COO]−, 133 | Coumestan | [30] |
| 51. | Trihydroxyflavanone | C15H12O5 | 11.639 | 271.0637 | 272.071 | −9.26 | 151, 119 | Flavanone | |
| 52. | Tetrahydroxyflavanone | C15H12O6 | 11.731 | 287.0583 | 288.0651 | −5.97 | 151, 135 | Flavanone | |
| 53. | Longusol C | C28H22O8 | 11.777 | 485.1269 | 486.133 | −3.11 | 375, 241, 177, 109 | Stilbene dimer | [31] |
| 54. | Hydroxy-methoxycoumarin | C10H8O4 | 11.822 | 191.0334 | 192.0409 | 7.11 | 176,148, 121,104 | Coumarin | |
| 55. | Trihydroxycinnamic acid dimethyl ether | C11H12O5 | 11.914 | 223.0634 | 224.0708 | −10.51 | 164, 146, 133, 117, 91 | Phenolic acid | |
| 56. | Tetrahydroxyflavone (Luteolin) | C15H10O6 | 12.279 | 285.0439 | 286.0512 | −12.14 | 199, 151, 135, 133 [M − C8H6O2 − H2O]−, 107 | Flavone | [32] |
| 57. | Dihydroxy-dimethoxyflavone (3′,4′-Dimethoxy luteolin) | C17H14O6 | 12.371 | 313.074 | 314.0812 | −7.03 | 298, 284, 254, | Flavone | |
| 58. | Trihydroxy-methoxyflavanone (Hesperitin) | C16H14O6 | 12.69 | 301.0742 | 302.0814 | −7.77 | 285, 165, 135 | Flavanone | |
| 59. | Tetrahydroxyflavanone | C15H12O6 | 12.736 | 287.0589 | 288.0661 | −9.41 | 151, 135 | Flavanone | |
| 60. | Tetrahydroxymethylaurone | C16H12O6 | 12.827 | 299.0589 | 300.0662 | −9.48 | 284 [M − H − CH3]−, 271 [M − H − CO]−, 179,165, 151, 135 | Aurone | [15] |
| 61. | Trihydroxyflavanone | C15H12O5 | 12.873 | 271.0641 | 272.0714 | −10.69 | 191, 151, 135 | Flavanone | |
| 62. | Hydroxymethoxycinnamaldehyde | C10H10O3 | 12.965 | 177.0574 | 178.0644 | −8.07 | 162 [M − H − CH3]−, 117 [M − H − OCH3]−, 91 (M − H − OCH3 − CO) | Aldehyde | |
| 63. | Trihydroxy-octadecadienoic acid | C18H32O5 | 13.193 | 327.2209 | 328.2283 | −10.04 | 299 [M − H − 28]−, 285, 229, 171, 85 | Fatty acid | [5] |
| 64. | Trihydroxy-methoxy methyl aurone | C17H14O6 | 13.33 | 313.0745 | 314.0817 | -8.56 | 298 [M − CH3]−, 283 [M − H − CHO), 270, 164, 149, 136, 121 | Aurone | [14,15] |
| 65. | Trihydroxy-octadecenoic acid | C18H34O5 | 13.559 | 329.2364 | 330.2439 | −9.8 | 311 [M − H − 18]−, 285 [M − H − 44]−, 229, 211, 171 | Fatty acid | |
| 66. | Tetrahydroxymethylaurone isomer | C16H12O6 | 13.787 | 299.059 | 300.066 | −8.79 | 284 [M − H − CH3]−, 271 [M − H − CO]−, 165, 151, 135 | Aurone | [15] |
| 67. | Trihydroxymethoxyprenyl isoflavone | C21H20O6 | 13.924 | 367.1226 | 368.1297 | −9.96 | 352 [M − H − CH3]−, 298 [M − H − C5H9]−, 269 [M − H − C5H9 − CHO]−, 165, 151, 135 | Isolavone | [33] |
| 68. | Tetrahydroxyflavanone methyl ether | C16H14O6 | 14.655 | 301.0748 | 302.082 | −9.82 | 285, 165, 135 | Flavanone | |
| 69. | Trihydroxy-prenylflavan | C20H22O4 | 14.747 | 325.1481 | 326.1549 | −9.61 | 311, 283, 249,241, 203, 183, 163, 145, 121, 109 | Flavan | |
| 70. | Trihydroxymethoxy prenylflavan | C21H24O5 | 14.929 | 355.1583 | 356.1658 | −9.68 | 321, 295 [M − OCH3]−, 267, 219, 149, 135, 97 | Flavan | [13,34] |
| 71. | Unknown steroid | C32H44O9 | 15.66 | 571.2934 | 572.3006 | −3.59 | 525, 315, 241, 153 | Steroid | |
| 72. | Unknown steroid | C26H34O4 | 16.392 | 409.2387 | 410.2454 | 0.86 | 315, 153 | Steroid | |
| 73. | Triterpene-Unidentified | C30H38O4 | 16.574 | 461.2701 | 462.2779 | −2.03 | 279, 153 | Triterpene | |
| 74. | Unknown steroid | C27H36O4 | 17.625 | 423.2552 | 424.2622 | -2.07 | 255, 153 | Steroid | |
| 75. | Triterpene-Unidentified | C30H38O4 | 17.717 | 461.2704 | 462.2776 | −1.36 | 279, 181 | Triterpene | |
| 76. | Unknown steroid | C26H34O4 | 19.362 | 409.2407 | 410.2476 | −4.68 | 279, 153, 79 | Steroid | |
| 77. | Triterpene-Unidentified | C30H38O4 | 20.093 | 461.2714 | 462.2787 | −3.73 | 279, 181, 125 | Triterpene |
| Parameters | Galactin-3 (ng/mL) | TNF-α Pg/mL | ||
|---|---|---|---|---|
| Groups | Mean ± S.E | % Change | Mean ± S.E | % Change |
| Control | 1.7 ± 0.14 | 0 | 30.61 ± 1.07 | 0 |
| Ethanol (1 mL) | 11.10 ± 0.85 a | +85 a | 212.71 ± 3.71 a | +85 a |
| Ranitidine + Ethanol | 2.06 ± 0.13 a b | −81.4 b | 44.25 ± 1.77 a,b | −79 b |
| Ex (25 mg) + Ethanol | 11.01 ± 0.46 a | −0.8 b | 183.15 ± 5.93 a,b | −13.9 b |
| Ex (50 mg) + Ethanol | 7.75 ± 0.29 a b | −30.4 b | 148.83 ± 5.75 a,b | −30 b |
| Ex (100 mg) + Ethanol | 5.63 ± 0.37 a b | −97.1 b | 128.98 ± 6.07 a,b | −39 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshamy, A.I.; Farrag, A.R.H.; Ayoub, I.M.; Mahdy, K.A.; Taher, R.F.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Al-Rejaie, S.S.; EI-Amier, Y.A.; Abd-EIGawad, A.M.; et al. UPLC-qTOF-MS Phytochemical Profile and Antiulcer Potential of Cyperus conglomeratus Rottb. Alcoholic Extract. Molecules 2020, 25, 4234. https://doi.org/10.3390/molecules25184234
Elshamy AI, Farrag ARH, Ayoub IM, Mahdy KA, Taher RF, Gendy AE-NGE, Mohamed TA, Al-Rejaie SS, EI-Amier YA, Abd-EIGawad AM, et al. UPLC-qTOF-MS Phytochemical Profile and Antiulcer Potential of Cyperus conglomeratus Rottb. Alcoholic Extract. Molecules. 2020; 25(18):4234. https://doi.org/10.3390/molecules25184234
Chicago/Turabian StyleElshamy, Abdelsamed I., Abdel Razik H. Farrag, Iriny M. Ayoub, Karam A. Mahdy, Rehab F. Taher, Abd El-Nasser G. EI Gendy, Tarik A. Mohamed, Salim S. Al-Rejaie, Yasser A. EI-Amier, Ahmed M. Abd-EIGawad, and et al. 2020. "UPLC-qTOF-MS Phytochemical Profile and Antiulcer Potential of Cyperus conglomeratus Rottb. Alcoholic Extract" Molecules 25, no. 18: 4234. https://doi.org/10.3390/molecules25184234
APA StyleElshamy, A. I., Farrag, A. R. H., Ayoub, I. M., Mahdy, K. A., Taher, R. F., Gendy, A. E. -N. G. E., Mohamed, T. A., Al-Rejaie, S. S., EI-Amier, Y. A., Abd-EIGawad, A. M., & Farag, M. A. (2020). UPLC-qTOF-MS Phytochemical Profile and Antiulcer Potential of Cyperus conglomeratus Rottb. Alcoholic Extract. Molecules, 25(18), 4234. https://doi.org/10.3390/molecules25184234