Assessment of Techno-Functional and Nutraceutical Potential of Tomato (Solanum lycopersicum) Seed Meal
Abstract
:1. Introduction
2. Results
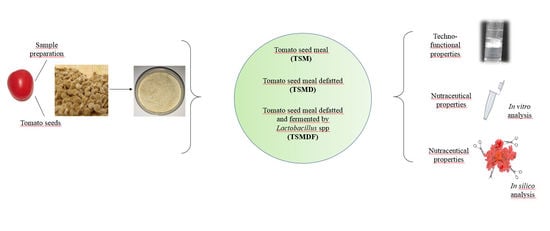
2.1. Sample Preparation and TSM Composition
2.2. Techno-Functional Properties
2.2.1. Water/Oil Holding Capacity: WHC and OHC
2.2.2. Protein Solubility
2.2.3. Foaming Capacity
2.2.4. Emulsifying Capacity
2.3. Nutraceutical Properties
2.4. Enhancement of Nutraceutical Properties by Fermentation
2.5. In Silico Analysis Biopeptides
3. Discussion
3.1. Techno-Functional Properties
3.2. Nutraceutical Properties
4. Materials and Methods
4.1. Sample Preparation
4.2. Proximal Composition, Phenolic Compounds, and Proteins
4.3. Techno-Functional Properties
4.3.1. Water Holding Capacity (WHC)
4.3.2. Oil Holding Capacity (OHC)
4.3.3. Protein Solubility (PS)
4.3.4. Foaming Properties
4.3.5. Emulsifying Properties
4.4. Nutraceutical Properties
4.4.1. Inhibitory Activity of the 2,2-diphenyl-1-picrylhydrazyl Radical Cation (DPPH)
4.4.2. Inhibitory Activity of the Radical Cation of 2,2′azinobis-(3-ethylbenzothialin)-6-sulfonic acid (ABTS)
4.4.3. Iron Chelating Activity
4.4.4. Angiotensin Converting Enzyme Inhibition (ACEI)
4.5. Enhancement of Nutraceutical Properties by Fermentation
4.6. In Silico Analysis: Biopeptides
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blanca, J.; Cañizares, J.; Cordero, L.; Pascual, L.; Diez, M.J.; Nuez, F. Variation revealed by SNP genotyping and morphology provides insight into the origin of the tomato. PLoS ONE 2012, 10, e48198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Faostat. Available online: http://www.fao.org/in-action/inpho/crop-compendium/cereals-grains/es/ (accessed on 10 July 2020).
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzyme Microb. Tech. 2011, 49, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, Z.; Venkitasamy, C.; Ma, H.; Li, Y. Umami taste amino acids produced by hydrolyzing extracted protein from tomato seed meal. LWT-Food Sci. Technol. 2015, 62, 1154–1161. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef] [Green Version]
- Soji, D.; Garg, S.; Bawa, A. Functional properties of seed meals and protein concentrates from tomato-processing waste. J. Food Sci. 2002, 2997–3001. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorization of tomato pomace: A comprehensive review. Trends Food Sci. Tech. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Abdollahzadeh, F.; Pirmohammadi, R.; Farhoomand, P.; Fatehi, F.; Pazhoh, F.F. The effect of ensiled mixed tomato and apple pomace on olstein dairy cow. Ital. J. Animal Sci. 2010, 9. [Google Scholar] [CrossRef]
- Gebeyew, K.; Animut, G.; Urge, M.; Feyera, T. The effect of feeding dried tomato pomace and concentrate feed on body weight change, carcass parameter and economic feasibility on hararghe highland sheep, Eastern Ethiopia. J. Vet. Sci. Tech. 2014, 6, 217. [Google Scholar] [CrossRef]
- Isik, F.; Topkaya, C. Effects of tomato pomace supplementation on chemical and nutritional properties of crackers. Ital. J. Food Sci. 2016, 28, 525–535. [Google Scholar] [CrossRef]
- Sogi, D.S.; Sidhu, J.S.; Arora, M.S.; Garg, S.K.; Bawa, A.S. Effect of tomato seed meal supplementation on the dough and bread characteristics of wheat (PBW343) flour. Int. J. Food Prop. 2002, 5, 563–571. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Setti, K.; Hamdi, M. Bioprocess development and preservation of functional food from tomato seed isolate fermented by kefir culture mixture. J. Food Sci. Tech. Mys 2018, 55, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Zayas, J.F. Solubility of Proteins. In Functionality of Proteins in Food, 1st. ed.; Zayas, J.F., Ed.; Springer: New York, NY, USA, 1997; pp. 6–75. [Google Scholar]
- Shao, D.; Atungulu, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Fan, Z. Characteristics of isolation and functionality of protein from tomato pomace produced with different industrial processing methods. Food Bioprocess Tech. 2014, 7, 532–541. [Google Scholar] [CrossRef]
- Hati, S.; Patel, N.; Sakure, A.; Mandal, S. Influence of whey protein concentrate on the production of antibacterial peptides derived from fermented milk by lactic acid bacteria. Int. J. Pept. Res. Ther. 2018, 24, 87–98. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Fagbemi, T.N.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. In vitro antihypertensive and antioxidative properties of alcalase-derived Moringa Oleifera seed globulin hydrolysate and its membrane fractions. J. Food Process Pres. 2019, 43, e13862. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Hamdi, M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31, 94–113. [Google Scholar] [CrossRef]
- Shao, D.; Atungulo, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Chen, X. Separation methods and chemical and nutritional characteristics of tomato pomace. T. ASABE 2013, 56, 261–268. [Google Scholar] [CrossRef]
- Kaur, D.; Sogi, D.S.; Garg, S.K.; Bawa, A.S. Flotation-cum-sedimentation system for skin and seed separation from tomato pomace. J. Food Eng. 2005, 71, 341–344. [Google Scholar] [CrossRef]
- Kang, S.W.; Rahman, M.S.; Kim, A.N.; Lee, K.Y.; Park, C.Y.; Kerr, W.L.; Choi, S.G. Comparative study of the quality characteristics of defatted soy flour treated by supercritical carbon dioxide and organic solvent. J. Food Sci. Technol. 2017, 54, 2485–2493. [Google Scholar] [CrossRef]
- Chandi, K.; Sogi, D. Functional properties of rice bran protein concentrates. J. Food Eng. 2007, 79, 592–597. [Google Scholar] [CrossRef]
- Awolu, O.O.; Osemeke, R.O.; Ifesan, B.O.T. Antioxidant, functional and rheological properties of optimized composite flour, consisting wheat and amaranth seed, brewers’ spent grain and apple pomace. J. Food Sci. Technol. 2016, 53, 1151–1163. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y. Composition, structure and functional properties of protein concentrates and isolates produced from walnut (Juglans regia L.). Int. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef] [Green Version]
- Ogunwolu, S.O.; Henshaw, F.O.; Mock, H.P.; Santros, A.; Awonorin, S.O. Functional properties of protein concentrates and isolates produced from cashewnut (Anacardium occidentale L.). Food Chem. 2009, 115, 852–858. [Google Scholar] [CrossRef]
- Kougias, P.G.; Tsapekos, P.; Boe, K.; Angelidaki, I. Antifoaming effect of chemical compounds in manure biogas reactors. Water Res. 2013, 47, 6280–6288. [Google Scholar] [CrossRef]
- Elsohaimy, S.A.; Refaay, T.M.; Zaytoun, M.A.M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Janssen, F.; Pauly, A.; Romboust, I.; Jansens, K.J.A.; Deleu, L.J.; Delcour, J.A. Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Comp. Rev. Food Sci. Saf. 2019, 16, 39–58. [Google Scholar] [CrossRef]
- Liu, M.; Lee, D.S.; Damodaran, S. Emulsifying properties of acidic subunits of soy 11S globulin. J. Agric. Food Chem. 1999, 47, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Luna-Suárez, S.; Medina-Godoy, S.; Cruz-Hernández, A.; Paredes-López, O. Modification of the amaranth 11S globulin storage protein to produce an inhibitory peptide of the Angiotensin I Converting Enzyme, and its expression in Escherichia coli. J. Biotechnol. 2010, 148, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Rana, J.C.; Kaur, A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int. J. Food Sci. Tech. 2014, 49, 541–550. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. Phenolic content and antioxidant and antimutagenic activities in tomato peel, seeds, and byproducts. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kaul, P. Evaluation of tomato processing by-products: A comparative study in a pilot scale setup. J. Food Process Eng. 2014, 299–307. [Google Scholar] [CrossRef]
- Kehrer, J. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Gunyakti, A.; Asan-Ozusaglam, M. Lactobacillus gasseri from human milk with probiotic potential and some technological properties. LWT 2019, 109, 261–269. [Google Scholar] [CrossRef]
- Chi, C.; Cho, S.J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2019, 68, 619–625. [Google Scholar] [CrossRef]
- Luz, C.; Izzo, L.; Graziani, G.; Gaspari, A.; Ritieni, A.; Mañes, J.; Meca, G. Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food Funct. 2018, 9, 3688–3697. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Walters, M.E.; Esfandi, R.; Tsopmo, A. Potential of food hydrolyzed proteins and peptides to chelate iron or calcium and enhance their absorption. Foods 2018, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Cheng, J.; Wu, H. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 15th ed.; AOAC International: Gaithersburg, MD, USA, 1990. [Google Scholar]
- Ayyash, M.; Johnson, S.K.; Liu, S.Q.; Mesmary, N.; Dahmani, S.; Al Dhaheri, A.S.; Kizhakkayil, J. In Vitro investigation of bioactivities of solid-state fermented lupin, quinoa and wheat using Lactobacillus spp. Food Chem. 2019, 275, 50–58. [Google Scholar] [CrossRef]
- Barbosa, H.; Slater, N.K.H.; Marcos, J.C. Protein quantification in the presence of poly (ethylene glycol) and dextran using the bradford method. Anal. Biochem. 2009, 395, 108–110. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.S.; de las Mercedes Salas-Mellado, M. How extraction method affects the physicochemical and functional properties of chia proteins. LWT 2018, 96, 26–33. [Google Scholar] [CrossRef]
- Prosekov, A.; Babich, O.; Kriger, O.; Ivanova, S.; Pavsky, V.; Sukhikh, S.; Yang, Y.; Kashirskih, E. Functional properties of the enzyme-modified protein from oat bran. Food Biosci. 2018, 24, 46–49. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Asmeret, A.B.; Teamrat, A.G. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Schomburg, I.; Chang, A.; Schomburg, D. BRENDA, enzyme data and metabolic information. Nucleic Acid. Res. 2002, 30, 47–49. [Google Scholar] [CrossRef]
Sample Availability: Samples of the seed meal are available from the authors. |




| Component | (%) |
|---|---|
| Moisture | 9.93 ± 0.73 |
| Ash | 3.92 ± 0.11 |
| Proteins | 26.93 ± 0.12 |
| Lipids | 14.02 ± 1.12 |
| Carbohydrates * | 18.21 |
| Fiber | 26.99 ± 2.92 |
| Techno-Functional Property | TSM | TSMD | ||||||
|---|---|---|---|---|---|---|---|---|
| pH 6 | pH 7 | pH 8 | pH 9 | pH 6 | pH 7 | pH 8 | pH 9 | |
| WHC * | 3.18 ± 0.11 a | 3.25 ± 0.25 a | 3.27 ± 0.11 a | 3.26 ± 0.05 a | 4.2 ± 0.2 b | 4.22 ± 0.23 b | 4.27 ± 0.17 b | 4.31 ± 0.31 b |
| OHC + | 2 ± 0.1 a | - | - | - | 2.3 ± 0.15 b | - | - | - |
| Protein solubility (%) | 41.6 ± 1.7 a | 45.7 ± 3.2 a | 50.1 ± 0.2 b | 55.9 ± 1.1 c | 64.2 ± 1.1 d | 70.5 ± 0.7 e | 79.4 ± 2.7 f | 83.7 ± 1.21 f |
| FA (%) « | <2.5 d | <2.5 d | <2.5 d | <2.5 d | 16.8 ± 0.3 a | 17.01 ± 0.04 a | 26.3 ± 0.13 b | 32.1 ± 0.2 c |
| Fst (min) ^ | <0.5 e | <0.5 e | <0.5 e | <0.5 e | 1.7 ± 0.2 a | 2.1 ± 0.01 b | 29.3 ± 0.5 c | 38.1 ± 0.1 d |
| EA (%) α | <4 h | 20 ± 0.01 a | 87.5 ± 0.06 b | 33.3 ± 0.02 c | 6.7 ± 0.02 d | 5.0 ± 0.03 e | 83.3 ± 0.07 f | 60.5 ± 0.06 g |
| Est (%) β | <1 h | 68.2 ± 0.01 a | 68.5 ± 0.04 b | 62.0 ± 0.05 c | 67.3 ± 0.09 d | 71.7 ± 1.1 e | 60.1 ± 0.07 f | 69.8 ± 0.1 g |
| Sample | DPPH | ABTS | Iron Chelating Activity | ACEI | ||
|---|---|---|---|---|---|---|
| μM Eq Trolox/100 g Sample | Scavenging Activity (% I) | μM Eq Trolox/100 g Sample | Scavenging Activity (% I) | (%) | Inhibition % | |
| TSM | 375.6 ± 4.6 a | 41.5 ± 2.3 a | 187.8 ± 2.2 a | 20.2 ± 0.3 a | 1.1 ± 0.3 a | 4.41 ± 1.3 a |
| TSMD | 355.1 ± 4.8 b | 38.9 ± 5.3 a | 177.3 ± 1.8 b | 19.3 ± 0.05 b | 2.2 ± 0.01 b | 7.9 ± 0.1 b |
| TSMDF (48 h) | 792.5 ± 5.3 c | 86.8 ± 1.3 b | 419.1 ± 6.5 c | 45.6 ± 0.2 c | 41.2 ± 1.1 c | 83.7 ± 0.7 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Torres, R.; Morales-Camacho, J.I.; López-Valdez, F.; Huerta-González, L.; Luna-Suárez, S. Assessment of Techno-Functional and Nutraceutical Potential of Tomato (Solanum lycopersicum) Seed Meal. Molecules 2020, 25, 4235. https://doi.org/10.3390/molecules25184235
Maldonado-Torres R, Morales-Camacho JI, López-Valdez F, Huerta-González L, Luna-Suárez S. Assessment of Techno-Functional and Nutraceutical Potential of Tomato (Solanum lycopersicum) Seed Meal. Molecules. 2020; 25(18):4235. https://doi.org/10.3390/molecules25184235
Chicago/Turabian StyleMaldonado-Torres, Ramón, Jocksan I. Morales-Camacho, Fernando López-Valdez, Luis Huerta-González, and Silvia Luna-Suárez. 2020. "Assessment of Techno-Functional and Nutraceutical Potential of Tomato (Solanum lycopersicum) Seed Meal" Molecules 25, no. 18: 4235. https://doi.org/10.3390/molecules25184235
APA StyleMaldonado-Torres, R., Morales-Camacho, J. I., López-Valdez, F., Huerta-González, L., & Luna-Suárez, S. (2020). Assessment of Techno-Functional and Nutraceutical Potential of Tomato (Solanum lycopersicum) Seed Meal. Molecules, 25(18), 4235. https://doi.org/10.3390/molecules25184235