Mononuclear Perfluoroalkyl-Heterocyclic Complexes of Pd(II): Synthesis, Structural Characterization and Antimicrobial Activity
Abstract
:1. Introduction
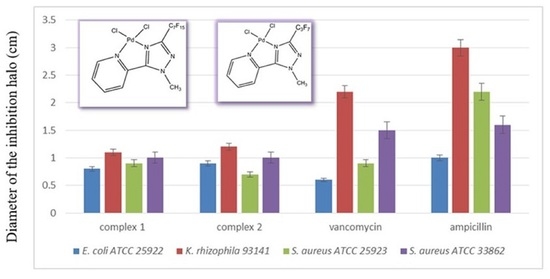
2. Results and Discussion
3. Materials and Methods
3.1. Instrumentation
3.2. Biological Sample and Reagents
3.3. Synthesis of Complex 1
3.4. Synthesis of Complex 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vitale, M.; Gaglio, S.; Galluzzo, P.; Cascone, G.; Piraino, C.; Di Marco Lo Presti, V.; Alduina, R. Antibiotic resistance profiling, analysis of virulence aspects and molecular genotyping of staphylococcus aureus isolated in sicily. Foodborne Pathog. Dis. 2018, 15, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.; Galluzzo, P.; Buffa, P.G.; Carlino, E.; Spezia, O.; Alduina, R. Comparison of antibiotic resistance profile and biofilm production of Staphylococcus aureus isolates derived from human specimens and animal-derived samples. Antibiotics 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is caretta caretta a carrier of antibiotic resistance in the mediterranean sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubino, S.; Pibiri, I.; Costantino, C.; Buscemi, S.; Girasolo, M.A.; Attanzio, A.; Tesoriere, L. Synthesis of platinum complexes with 2-(5-perfluoroalkyl-1,2,4-oxadiazol-3yl)-pyridine and 2-(3-perfluoroalkyl-1-methyl-1,2,4-triazole-5yl)-pyridine ligands and their in vitro antitumor activity. J. Inorg. Biochem. 2016, 155, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Rubino, S.; Pibiri, I.; Minacori, C.; Alduina, R.; Di Stefano, V.; Orecchio, S.; Buscemi, S.; Girasolo, M.A.; Tesoriere, L.; Attanzio, A. Synthesis, structural characterization, anti-proliferative and antimicrobial activity of binuclear and mononuclear Pt(II) complexes with perfluoroalkyl-heterocyclic ligands. Inorg. Chim. Acta 2018, 483, 180–190. [Google Scholar] [CrossRef]
- Rubino, S.; Di Stefano, V.; Attanzio, A.; Tesoriere, L.; Girasolo, M.A.; Nicolò, F.; Bruno, G.; Orecchio, S.; Stocco, G.C. Synthesis, spectroscopic characterization and antiproliferative activity of two platinum(II) complexes containing N-donor heterocycles. Inorg. Chim. Acta 2014, 418, 112–118. [Google Scholar] [CrossRef]
- Rubino, S.; Saladino, M.L.; Busà, R.; Chillura Martino, D.F.; Girasolo, M.A.; Caponetti, E.; Tesoriere, L.; Attanzio, A. Loading and release of the complex [Pt(DTBTA)(DMSO)Cl]Cl·CHCl3 with the 2,2′-dithiobis(benzothiazole) ligand into mesoporous silica and studies of antiproliferative activity on MCF-7 cells. Polyhedron 2018, 153, 234–239. [Google Scholar] [CrossRef]
- Mo, Q.Y.; Deng, J.G.; Liu, Y.; Huang, G.D.; Li, Z.W.; Yu, P.; Gou, Y.; Yang, F. Mixed-ligand Cu(II) hydrazone complexes designed to enhance anticancer activity. Eur. J. Med. Chem. 2018, 156, 368–380. [Google Scholar] [CrossRef]
- Simovic’, A.R.; Masnikosa, R.; Bratsos, I.; Alessio, E. Chemistry and reactivity of ruthenium(II) complexes: DNA/protein binding mode and anticancer activity are related to the complex structure. Coord. Chem. Rev. 2019, 398, 113011. [Google Scholar] [CrossRef]
- Bhatti, M.Z.; Ali, A.; Duongd, H.Q.; Chen, J.; Rahmanf, F.U. Anticancer activity and mechanism of bis-pyrimidine based dimetallic Ru(II)(η6-p-cymene) complex in human non-small cell lung cancer via p53-dependent pathway. J. Inorg. Biochem. 2019, 194, 52–64. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Tesoriere, L.; Casella, G.; Attanzio, A.; Capobianco, M.L.; Sabatino, P.; Barone, G.; Rubino, S.; Bonsignore, R. A novel compound of triphenyltin(IV) with N-tert-butoxycarbonyl-l-ornithine causes cancer cell death by inducing a p53-dependent activation of the mitochondrial pathway of apoptosis. Inorg. Chim. Acta 2017, 456, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Attanzio, A.; D’Agostino, S.; Busà, R.; Frazzitta, A.; Rubino, S.; Girasolo, M.A.; Sabatino, P.; Tesoriere, L. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules 2020, 25, 859. [Google Scholar] [CrossRef] [Green Version]
- Petrović, B.; Jovanović, S.; Puchta, R.; van Eldik, R. Mechanistic insight on the chemistry of potential Pt antitumor agents as revealed by collaborative research performed in Kragujevac and Erlangen. Inorg. Chim. Acta 2019, 495, 118953. [Google Scholar] [CrossRef]
- Fanelli, M.; Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M.; Paoli, P. New trends in platinum and palladium complexes as antineoplastic agents. Coord. Chem. Rev. 2016, 310, 41–79. [Google Scholar] [CrossRef]
- Rubino, S.; Busà, R.; Attanzio, A.; Alduina, R.; Di Stefano, V.; Girasolo, M.A.; Orecchio, S.; Tesoriere, L. Synthesis, properties, antitumor and antibacterial activity of new Pt(II) and Pd(II) complexes with 2,2′-dithiobis(benzothiazole) ligand. Bioorg. Med. Chem. 2017, 25, 2378–2386. [Google Scholar] [CrossRef] [Green Version]
- Pages, B.J.; Sakoff, J.; Gilbert, J.; Zhang, Y.; Preston, D.; Li, F.; Crowley, J.D.; Aldrich-Wright, J.R. Investigating the cytotoxicity of platinum(II) complexes incorporating bidentate pyridyl-1,2,3-triazole “click” ligands. J. Inorg. Biochem. 2016, 165, 92–99. [Google Scholar] [CrossRef]
- Łakomska, I.; Barwiołek, M.; Wojtczak, A.; Szłyk, E. X-ray structure and multinuclear NMR studies of platinum(II) and palladium(II) complexes with 5,7-ditertbutyl-1,2,4-triazolo[1,5-a]pyrimidine. Polyhedron 2007, 26, 5349–5354. [Google Scholar] [CrossRef]
- Bharty, M.K.; Pswan, S.; Dani, R.K.; Singh, N.K.; Sharma, V.K.; Kharwar, R.N. Polymeric Cd(II), trinuclear and mononuclear Ni(II) complexes of 5-methyl-4-phenyl-1,2,4-triazole-3-thione: Synthesis, structural characterization, thermal behavior, fluorescence properties and antibacterial activity. J. Mol. Struct. 2017, 1130, 181–193. [Google Scholar] [CrossRef]
- Kilpin, K.J.; Gavey, E.L.; McAdam, C.J.; Anderson, C.B.; Lind, S.J.; Keep, C.C.; Gordon, K.C.; Crowley, J.D. Palladium(II) Complexes of Readily Functionalized Bidentate 2-Pyridyl-1,2,3-triazole “Click” Ligands: A Synthetic, Structural, Spectroscopic, and Computational Study. Inorg. Chem. 2011, 50, 6334–6346. [Google Scholar] [CrossRef]
- Mekni, N.H. A simple and efficient intramolecular 1,3-dipolar azidoalkyne cycloaddition: Synthesis of 6-perfluoroalkylated fused exo-bicyclic 1,2,3-triazolo-1,4-oxazines. J. Fluor. Chem. 2016, 186, 97–100. [Google Scholar] [CrossRef]
- Brown, H.C.; Kassal, R.J. 5-Perfluoroalkyltetrazoles. I. Ring-opening reactions. J. Org. Chem. 1967, 32, 1871–1873. [Google Scholar] [CrossRef]
- Grünebaum, M.; Gerlitz, A.I.; Buchheit, A.; Jeschke, S.; Daniliuc, C.G.; Wiemhöfer, H.D. Improved synthesis of perfluoroalkyl substituted 1,3,4-oxadiazoles as precursors for corresponding 1,2,4-triazoles. J. Fluor. Chem. 2016, 183, 30–35. [Google Scholar] [CrossRef]
- Riccobono, A.; Slattery, J.M.; Parker, R.R.; Whitwood, A.C.; Bruce, D.W.; Pibiri, I.; Pace, A. 1,2,4-Triazolium ions as flexible scaffolds for the construction of polyphilic ionic liquid crystals. Chem. Commun. 2018, 54, 9965–9968. [Google Scholar] [CrossRef] [Green Version]
- Pibiri, I.; Buscemi, S. A Recent Portrait of Bioactive Triazoles. Curr. Bioact. Comp. 2010, 6, 208–282. [Google Scholar] [CrossRef]
- Rubino, S.; Portanova, P.; Albanese, A.; Calvaruso, G.; Orecchio, S.; Fontana, G.; Stocco, G.C. Mono- and polynuclear complexes of Pt(II) with polypyridyl ligands: Synthesis, spectroscopic and structural characterization and cytotoxic activity. J. Inorg. Biochem. 2007, 101, 1473–1482. [Google Scholar] [CrossRef]
- Łakomska, I.; Hoffmann, K.; Muzioł, T.; Sitkowski, J. Multinuclear magnetic resonance and X-ray characterization of platinum(II) complexes with substituted-1,2,4-triazolo[1,5-a]pyrimidines. J. Mol. Struct. 2014, 1056, 146–151. [Google Scholar] [CrossRef]
- Łakomska, I.; Babinska, M.; Wojtczak, A.; Sitkowski, J. Synthesis, characterization and in vitro cytotoxicity of three types of platinum(II) complexes containing 5,7-diethyl-1,2,4-triazolo[1,5-a]pyrimidine. Inorg. Chim. Acta 2016, 453, 516–521. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; Zito, P.; Alduina, R.; Ramarosandratana, A.V.; Bruno, M.; Rosselli, S.; Sajeva, M.; Notarbartolo, M. Essential Oil Composition of Alluaudia procera and in Vitro Biological Activity on Two Drug-Resistant Models. Molecules 2019, 24, 2871. [Google Scholar] [CrossRef] [Green Version]
- Ciabocco, M.; Cancemi, P.; Saladino, M.L.; Caponetti, E.; Alduina, R.; Berrettoni, M. Synthesis and antibacterial activity of iron-hexacyanocobaltate nanoparticles. J. Biol. Inorg. Chem. 2018, 23, 385–398. [Google Scholar] [CrossRef]
- Saladino, M.L.; Markowska, M.; Carmone, C.; Cancemi, P.; Alduina, R.; Presentato, A.; Scaffaro, R.; Biały, D.; Hasiak, M.; Hreniak, D.; et al. Graphene Oxide Carboxymethylcellulose Nanocomposite for Dressing Materials. Materials 2020, 13, 1980. [Google Scholar] [CrossRef]
- Raimondi, M.R.V.; Presentato, A.; Li Petri, G.; Buttacavoli, M.; Ribaudo, A.; Alduina, R.; Cancemi, P. New synthetic nitro-pyrrolomycins as promising antibacterial and anticancer agents. Antibiotics 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.F.; Migliore, L.; Mattei, D.; Rotini, A.; Thaller, M.C.; Alduina, R. Antibiotic Resistance of Gram-Negative Bacteria from Wild Captured Loggerhead Sea Turtles. Antibiotics 2020, 9, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the Ability of Perfluorohexane Sulfonate (PFHxS) Bioaccumulation by Two Pseudomonas sp. Strains Isolated from PFAS-Contaminated Environmental Matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presentato, A.; Cappelletti, M.; Sansone, A.; Ferreri, C.; Piacenza, E.; Demeter, M.A.; Crognale, S.; Petruccioli, M.; Milazzo, G.; Fedi, S.; et al. Aerobic Growth of Rhodococcus aetherivorans BCP1 Using Selected Naphthenic Acids as the Sole Carbon and Energy Sources. Front. Microbiol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Wójcik, A.; Perczyk, P.; Wydro, P.; Broniatowski, M. Effects of water soluble perfluorinated pollutants on phospholipids in model soil decomposer membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Schöniger, W. Eine mikroanalytische Schnellbestimmung von Halogen in organischen Substanzen. Mikrochim. Acta 1955, 43, 123–129. [Google Scholar] [CrossRef]
- Dresler, C.; Saladino, M.L.; Demirbag, C.; Caponetti, E.; Chillura Martino, D.F.; Alduina, R. Development of controlled release systems of biocides for the conservation of cultural heritage. Int. Biodeterior. Biodegrad. 2017, 125, 150–156. [Google Scholar] [CrossRef]
- Russo, M.; La Corte, D.; Pisciotta, A.; Riela, S.; Alduina, R.; Lo Meo, P. Binding abilities of polyaminocyclodextrins: Polarimetric investigations and biological assays. Beilstein J. Org. Chem. 2017, 13, 2751–2763. [Google Scholar] [CrossRef]
- Pibiri, I.; Pace, A.; Buscemi, S.; Vivona, N.; Malpezzi, L. Designing fluorous domains synthesis of a series of pyridinium salts bearing a perfluoralkylated azole moiety. Heterocycles 2006, 68, 307–321. [Google Scholar]
- Pace, A.; Palumbo Piccionello, A.; Pibiri, I.; Accardo, A.; Vivona, N.; Buscemi, S. Applications of Ring Rearrangements Involving a Participating Side Chain for the Synthesis of Five-Membered Heterocycles. Targets Heterocycl. Syst. 2014, 18, 48–86. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Pace, A.; Pibiri, I.; Vivona, N.; Spinelli, D. Fluorinated Heterocyclic Compounds. An Expedient Route to 5-Perfluoroalkyl-1,2,4-triazoles via an Unusual Hydrazinolysis of 5-Perfluoroalkyl-1,2,4-oxadiazoles: First Examples of an ANRORC-Like Reaction in 1,2,4-Oxadiazole Derivatives. J. Org. Chem. 2003, 68, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds [PdCl2(pfptp)] (1) and [PdCl2(pfhtp)] (2) are available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubino, S.; Alduina, R.; Cancemi, P.; Girasolo, M.A.; Di Stefano, V.; Orecchio, S.; Buscemi, S.; Pibiri, I. Mononuclear Perfluoroalkyl-Heterocyclic Complexes of Pd(II): Synthesis, Structural Characterization and Antimicrobial Activity. Molecules 2020, 25, 4487. https://doi.org/10.3390/molecules25194487
Rubino S, Alduina R, Cancemi P, Girasolo MA, Di Stefano V, Orecchio S, Buscemi S, Pibiri I. Mononuclear Perfluoroalkyl-Heterocyclic Complexes of Pd(II): Synthesis, Structural Characterization and Antimicrobial Activity. Molecules. 2020; 25(19):4487. https://doi.org/10.3390/molecules25194487
Chicago/Turabian StyleRubino, Simona, Rosa Alduina, Patrizia Cancemi, Maria Assunta Girasolo, Vita Di Stefano, Santino Orecchio, Silvestre Buscemi, and Ivana Pibiri. 2020. "Mononuclear Perfluoroalkyl-Heterocyclic Complexes of Pd(II): Synthesis, Structural Characterization and Antimicrobial Activity" Molecules 25, no. 19: 4487. https://doi.org/10.3390/molecules25194487
APA StyleRubino, S., Alduina, R., Cancemi, P., Girasolo, M. A., Di Stefano, V., Orecchio, S., Buscemi, S., & Pibiri, I. (2020). Mononuclear Perfluoroalkyl-Heterocyclic Complexes of Pd(II): Synthesis, Structural Characterization and Antimicrobial Activity. Molecules, 25(19), 4487. https://doi.org/10.3390/molecules25194487