Spectroscopic and In Vitro Investigations of Boron(III) Complex with Meso-4-Methoxycarbonylpropylsubstituted Dipyrromethene for Fluorescence Bioimaging Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of BODIPY
2.2. X-Ray Data
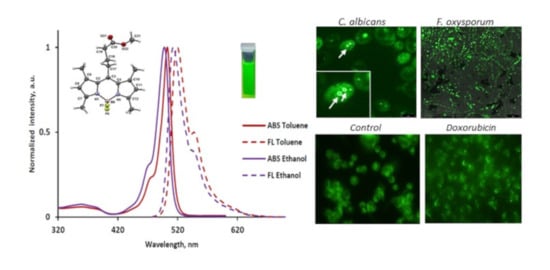
2.3. Spectroscopic Properties
2.4. Partition Coefficient Determination
2.5. Photostability
2.6. Fluorescent Staining of Pathogenic Microorganisms
2.7. Fluorescent Staining of Mammalian Cells
3. Experimental
3.1. Chemistry
3.2. NMR Studes
3.3. MALDI TOF Studes
3.4. Elemental Analyses
3.5. UV-Vis Spectroscopy
3.6. X-ray Structure Determinations of [BF2L]
3.7. Biological Studies
3.8. Confocal Laser Scanning Microscopy
3.9. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perez-Nadales, E.; Nogueira, M.F.A.; Baldin, C.; Castanheira, S.; Ghalid, M.E.; Grund, E.; Lengeler, K.; Marchegiani, E.; Pankaj Vinod Mehrotra, P.V.; Moretti, M.; et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Gen. Biol. 2014, 70, 42–67. [Google Scholar] [CrossRef] [Green Version]
- Solomonov, A.V.; Marfin, Y.S.; Rumyantsev, E.V. Design and applications of dipyrrin-based fluorescent dyes and related organic luminophores: From individual compounds to supramolecular selfassembled systems. Dyes Pig. 2019, 162, 517–542. [Google Scholar] [CrossRef]
- Okamoto, M.; Kabayashi, S.; Ikeuchi, H.; Yamada, S.; Yamanouchi, K.; Nagasawa, K.; Maekawa, S.; Kato, T.; Shimizu, I. Synthesis and bioassay of a boron-dipyrromethene derivative of estradiol for fluorescence imaging in vivo. Steroids 2012, 77, 845–849. [Google Scholar] [CrossRef]
- Jantra, S.; Butta, P.; Jithavech, P.; Rojsitthisak, P.; Palaga, T.; Rashatasakhon, P.; Sukwattanasinitt, M.; Wacharasindhu, S. Turn on” orange fluorescent probe based on styryl-BODIPY for detection of hypochlorite and its application in live cell imaging. Dyes Pig. 2019, 162, 189–195. [Google Scholar] [CrossRef]
- West, R.; Panagabko, C.; Atkinson, J. Synthesis and characterization of BODIPY-α-tocopherol: A fluorescent form of vitamin E. J. Org. Chem. 2010, 75, 2883–2892. [Google Scholar] [CrossRef] [Green Version]
- Alekseeva, A.S.; Tretiakova, D.S.; Melnikova, D.N.; Molotkovsky, U.G.; Boldyrev, I.A. Novel fluorescent membrane probe 2,3,5,6-bis(cyclohexyl)-BODIPY-labeled phosphatidylcholine. Russ. J. Bioorg. Chem. 2016, 42, 305–309. [Google Scholar] [CrossRef]
- Gabe, Y.; Urano, Y.; Kikuchi, K.; Kojima, H.; Nagano, T. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophores rational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 2004, 126, 3357–3367. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Peng, X.; Cui, A.; Wu, Y.; Tian, M.; Zhang, L.; Chen, X.; Gao, Y. Synthesis and spectral properties of new boron dipyrromethene dyes. Dyes Pig. 2007, 73, 206–210. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, L.; Zhang, D.; Bian, Y.; Jiang, J. Modulation of the spectroscopic property of Bodipy derivates through tuning the molecular configuration. Photochem. Photobiol. Sci. 2011, 10, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.G.; Xia, J.X.; Fang, K.; Zhao, Q.H.; Wu, J.H.; Dai, J.C. A simple BODIPY-aniline-based fluorescent chemosensor as multiple logic operations for the detection of pH and CO2 gas. Dalt. Trans. 2014, 43, 8499–8507. [Google Scholar]
- Boldyrev, J.; Molotkovsky, G. Synthesis and properties of new 4,4-difluoro-3a,4a-diazas-indacene (BODIPY)-labeled lipids. Bioorgan. Khim. 2006, 32, 78–83. [Google Scholar] [CrossRef]
- Pakhomov, A.A.; Kononevich, Y.N.; Stukalova, M.V.; Svidchenko, E.A.; Surin, N.M.; Cherkaev, G.V.; Shchegolikhina, O.I.; Martynov, V.I.; Muzafarov, A.M. Synthesis and photophysical properties of a new BODIPY-basedsiloxane dye. Tetrahed. Lett. 2016, 57, 979–982. [Google Scholar] [CrossRef]
- Machida, S.; Tsubamoto, M.; Kato, N.; Harada, K.; Ohkanda, J. Peptidomimetic modification improves cell permeation of bivalentfarnesyltransferase inhibitors. Bioorg. Med. Chem. 2013, 21, 4004–4010. [Google Scholar] [CrossRef] [PubMed]
- Bröring, M.; Krüger, R.; Link, S.; Kleeberg, C.; Köhler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2′-bidipyrrins (bisBODIPYs): Highly fluorescent BODIPY dimers with large stokes shifts. J. Chem. Eur. 2008, 14, 2976–2983. [Google Scholar] [CrossRef]
- Yin, X.D.; Li, Y.J.; Zhu, Y.L.; Jing, X.; Li, Y.L.; Zhu, D.B. A highly sensitive viscosity probe based on ferrocene-BODIPY dyads. Dalt. Trans. 2010, 39, 9929–9935. [Google Scholar] [CrossRef] [PubMed]
- Zhong-Hua, P.; Jing-Wei, Z.; Geng-Geng, L. Experimental and theoretical study of enol–keto prototropic tautomerism and photophysics of azomethine–BODIPY dyads. Phys. Chem. 2014, 16, 16290–16301. [Google Scholar]
- Thivierge, C.; Han, J.; Jenkins, R.M.; Burgess, K. Fluorescent proton sensors based on energy transfer. J. Org. Chem. 2011, 76, 5219–5228. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Fan, J.; Gao, X.; Wang, B.; Sun, S.; Peng, X. Carboxyl BODIPY dyes from bicarboxylic anhydrides: One-pot preparation, spectral properties, photostability, and biolabeling. J. Org. Chem. 2009, 74, 7675–7683. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V.; Berezin, M.B.; Kalyagin, A.A. Influence of structural and solvation factors on the spectral-fluorescent properties of alkyl-substituted BODIPYs in solutions. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2017, 173, 228–234. [Google Scholar] [CrossRef]
- Nuraneeva, E.N.; Antina, E.V.; Guseva, G.B.; Berezin, M.B.; V’yugin, A.I. Effects of halogen substitution on the photostability and thermal degradation of boron(III), zinc(II) and cadmium(II) dipyrrinato complexes. Inorgan. Chim. Acta 2018, 482, 800–806. [Google Scholar] [CrossRef]
- Guseva, G.B.; Antina, E.V.; Berezin, M.B.; Pavelyev, R.S.; Kayumov, A.R.; Sharafutdinov, I.S.; Lisovskaya, S.A.; Lodochnikova, O.A.; Islamov, D.R.; Usachev, K.S.; et al. Meso-substituted-BODIPY based fluorescent biomarker: Spectral characteristics, photostability and possibilities for practical application. J. Photochem. Photobiol. Chem. 2020, 401, 112783. [Google Scholar] [CrossRef]
- Kritskaya, A.Y.; Berezin, M.B.; Antina, E.V.; Vyugin, A.I. Effect of aryl-, halogen-, and ms-aza-substitution on the luminescent properties and photostability of difluoroborates of 2,2′-dipyrrometenes. J. Fluores. 2019, 29, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Guseva, G.B.; Antina, E.V.; Beresin, M.B.; V’Yugin, A.I.; Nuraneeva, E.N. Preparation, spectral and thermal properties of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) complexes with iodosubstituted 2,2′-dipyrrolylmethene. Russ. J. Gen. Chem. 2013, 83, 1571–1597. [Google Scholar] [CrossRef]
- Guseva, G.B.; Antina, E.V.; Ksenofontov, A.A.; Nuraneeva, E.N. A quantum chemical study of the molecular structure of zinc(II) and boron(III) complexes with monoiodo and dibromo substituted dipyrrines. J. Struct. Chem. 2016, 57, 25–32. [Google Scholar] [CrossRef]
- Porter, G.; West, M.A. Flash Photolysis Techniques of Chemistry; Weissberger, A., Hammes, G.G., Eds.; Wiley-Inters: New York, NY, USA, 1974; Volume 4, pp. 367–462. [Google Scholar]
- Wang, X.; Zhou, Y.; Yu, W.; Wang, C.; Fang, Q.; Jiang, M.; Lei, H.; Wang, H. Two-photon pumped lasing stilbene-type chromophores containing various terminal donor groups: Relationship between lasing efficiency and intramolecular charge transfer. J. Mater. Chem. 2000, 10, 2698–2703. [Google Scholar] [CrossRef]
- Rella, A.; Farnoud, A.M.; Poeta, M.D. Plasma Membrane Lipids and Their Role in Fungal Virulence. Prog. Lipid Res. 2016, 61, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Georges, J. Fluorescence quantum yield of rhodamine 6G in ethanol as a function of concentration using thermal lens spectrometry. Chem. Phys. Lett. 1996, 260, 115–118. [Google Scholar] [CrossRef]
- Ping, C.; Shuqing, S.; Yunfeng, H.; Zhiguo, Q.; Deshui, Z. Structure and solvent effect on the photostability of indolenine cyanine dyes. Dyes Pigm. 1999, 41, 227–231. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT: Integrating space group determination and structure solution. Acta Cryst. Sect. Found. Adv. 2014, 70, 1437–1442. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallographica Section A: Foundations of Crystallography. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, T.; Sonobe, H.; Toyonaga, S.; Yamasaki, I.; Shuin, T.; Takano, A.; Araki, K.; Akimaru, K.; Yuri, K. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab. Investig. 2002, 82, 663–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boichuk, S.; Galembikova, A.; Dunaev, P.; Valeeva, E.; Shagimardanova, E.; Gusev, O.; Khaiboullina, S. A novel receptor tyrosine kinase switch promotes gastrointestinal stromal tumor drug resistance. Molecules 2017, 22, 2152. [Google Scholar] [CrossRef] [Green Version]
- Sharafutdinov, I.S.; Pavlova, A.S.; Akhatova, F.S.; Khabibrakhmanova, A.M.; Rozhina, E.V.; Romanova, Y.J.; Fakhrullin, R.; Lodochnikova, O.A.; Kurbangalieva, A.R.; Bogachev, M.I.; et al. Unraveling the Molecular Mechanism of Selective Antimicrobial Activity of 2(5H)-Furanone Derivative against Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 694. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Experimental resources are available from the authors. |






| Solvent | λ, nm (ε, L·mol−1·cm−1) S0 → S1 S0 → S2 | λ, nm (λex = 470 nm) S1→S0 | Δνst, cm−1 | φ (λex = 470 nm) S1 → S0 | krad·10−7, s−1 | knr·10−7, s−1 | τ, ns |
|---|---|---|---|---|---|---|---|
| cyclohexane | 502 (72,772) 476(sh) 358–361 | 516 | 541 | 1.000 | 8.33 | 0 | 12.0 |
| toluene | 503(70,323) 472(sh) 359–361 | 520 | 650 | 0.979 | 6.83 | 0.14 | 14.3 |
| chloroform | 502(66,317) 472(sh) 359–362 | 517 | 578 | 0.972 | 6.84 | 0.20 | 14.2 |
| octanol-1 | 502(61,467) 472(sh) 358–364 | 516 | 541 | 0.969 | 6.06 | 0.19 | 16.0 |
| butanol-1 | 499(59,849) 472(sh) 358–360 | 514 | 585 | 0.949 | 6.30 | 0.34 | 15.1 |
| ethanol | 498(60,491) 472(sh) 358–360 | 513 | 587 | 0.799 | 6.39 | 1.61 | 12.5 |
| DMSO | 498(56,469) 471(sh) 359–362 | 515 | 663 | 0.749 | 5.96 | 2.02 | 12.6 |
| Complexes | Solvent | t1/2, h | kobs ·10−6, s−1 |
|---|---|---|---|
 Difluoroborate 3,3′,5,5′-tetramethyl-2,2′-dipyrrometene [22] | cyclohexane toluene | 46.0 16.7 | 3.3 ± 0.1 8.6 ± 0.6 |
 | cyclohexane toluene | 88.6 40.5 | 2.9 ± 0.2 5.5 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guseva, G.; Antina, E.; Berezin, M.; Lisovskaya, S.; Pavelyev, R.; Kayumov, A.; Lodochnikova, O.; Islamov, D.; Usachev, K.; Boichuk, S.; et al. Spectroscopic and In Vitro Investigations of Boron(III) Complex with Meso-4-Methoxycarbonylpropylsubstituted Dipyrromethene for Fluorescence Bioimaging Applications. Molecules 2020, 25, 4541. https://doi.org/10.3390/molecules25194541
Guseva G, Antina E, Berezin M, Lisovskaya S, Pavelyev R, Kayumov A, Lodochnikova O, Islamov D, Usachev K, Boichuk S, et al. Spectroscopic and In Vitro Investigations of Boron(III) Complex with Meso-4-Methoxycarbonylpropylsubstituted Dipyrromethene for Fluorescence Bioimaging Applications. Molecules. 2020; 25(19):4541. https://doi.org/10.3390/molecules25194541
Chicago/Turabian StyleGuseva, Galina, Elena Antina, Mikhail Berezin, Svetlana Lisovskaya, Roman Pavelyev, Airat Kayumov, Olga Lodochnikova, Daut Islamov, Konstantin Usachev, Sergei Boichuk, and et al. 2020. "Spectroscopic and In Vitro Investigations of Boron(III) Complex with Meso-4-Methoxycarbonylpropylsubstituted Dipyrromethene for Fluorescence Bioimaging Applications" Molecules 25, no. 19: 4541. https://doi.org/10.3390/molecules25194541
APA StyleGuseva, G., Antina, E., Berezin, M., Lisovskaya, S., Pavelyev, R., Kayumov, A., Lodochnikova, O., Islamov, D., Usachev, K., Boichuk, S., & Nikitina, L. (2020). Spectroscopic and In Vitro Investigations of Boron(III) Complex with Meso-4-Methoxycarbonylpropylsubstituted Dipyrromethene for Fluorescence Bioimaging Applications. Molecules, 25(19), 4541. https://doi.org/10.3390/molecules25194541