A New Look into the Mode of Action of Metal-Based Anticancer Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Drug-DNA Samples
2.2. FTIR-ATR
2.3. MicroRaman
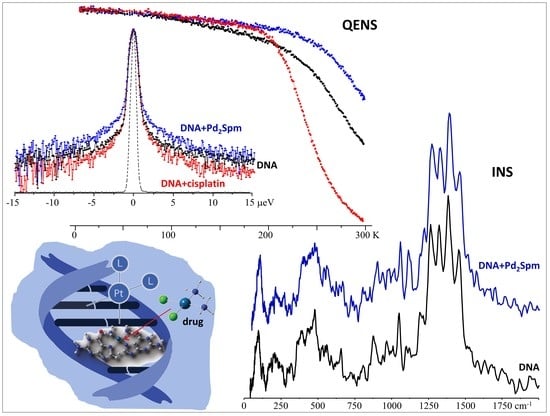
2.4. INS
2.5. QENS
3. Results and Discussion
3.1. Drug Effect on DNA Conformation
3.2. Drug Effect on DNA Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, B.; Vancamp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The Status of Platinum Anticancer Drugs in the Clinic and in Clinical Trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.P.M. Platinum and Palladium Polyamine Complexes as Anticancer Agents: The Structural Factor. ISRN Spectrosc. 2013, 2013, 1–29. [Google Scholar] [CrossRef]
- Alam, M.N.; Huq, F. Comprehensive Review on Tumour Active Palladium Compounds and Structure-Activity Relationships. Coord. Chem. Rev. 2016, 316, 36–67. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Amado, A.M.; Oliveira, P.J.; Sardao, V.A.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Pt(II) vs. Pd(II) polyamine complexes as new anticancer drugs: A structure-activity study. Lett. Drug Des. Discov. 2006, 3, 149–151. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.S.; Fiuza, S.M.; Goncalves, M.J.; Batista de Carvalho, L.A.E.; Marques, M.P.M.; Urbano, A.M. Effect of the metal center on the antitumor activity of the analogous dinuclear spermine chelates (PdCl2)2(spermine) and(PtCl2)2(spermine). Lett. Drug Des. Discov. 2007, 4, 460–463. [Google Scholar] [CrossRef] [Green Version]
- Fiuza, S.M.; Holy, J.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Biologic Activity of a Dinuclear Pd(II)-spermine Complex Toward Human Breast Cancer. Chem. Biol. Drug Des. 2011, 77, 477–488. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.M.; Fiuza, S.M.; Marques, M.P.M.; Persson, L.; Oredsson, S. Increased Breast Cancer Cell Toxicity by Palladination of the Polyamine Analogue N-1,N-11-bis(ethyl)norspermine. Amino. Acids 2014, 46, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Batista de Carvalho, A.L.M.; Medeiros, P.S.C.; Costa, F.M.; Ribeiro, V.P.; Sousa, J.B.; Diniz, C.; Marques, M.P.M. Anti-Invasive and Anti-Proliferative Synergism between Docetaxel and a Polynuclear Pd-Spermine Agent. PLoS ONE 2016, 11, e0167218. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.P.M.; Gianolio, D.; Cibin, G.; Tomkinson, J.; Parker, S.F.; Valero, R.; Lopes, R.P.; Batista de Carvalho, L.A.E. A Molecular View of Cisplatin’s Mode of Action: Interplay with DNA Bases and Acquired Resistance. Phys. Chem. Chem. Phys. 2015, 17, 5155–5171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista de Carvalho, A.L.M.; Mamede, A.P.; Dopplapudi, A.; Garcia Sakai, V.; Doherty, J.; Frogley, M.; Cinque, G.; Gardner, P.; Gianolio, D.; Batista de Carvalho, L.A.E.; et al. Anticancer Drug Impact on DNA—A Study by Neutron Spectroscopy Coupled with Synchrotron-based FTIR and EXAFS. Phys. Chem. Chem. Phys. 2019, 21, 4162–4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista de Carvalho, A.L.M.; Pilling, M.; Gardner, P.; Doherty, J.; Cinque, G.; Wehbe, K.; Kelley, C.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Chemotherapeutic Response to Cisplatin-like Drugs in Human Breast Cancer Cells Probed by Vibrational Microspectroscopy. Farad Discuss. 2016, 187, 273–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamego, I.; Marques, M.P.M.; Duarte, I.F.; Martins, A.S.; Oliveira, H.; Gil, A.M. Impact of the Pd2Spermine Chelate on Osteosarcoma Metabolism: An NMR Metabolomics Study. J. Proteome Res. 2017, 16, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.P.M.; Batista de Carvalho, A.L.M.; Sakai, V.G.; Hatter, L.; Batista de Carvalho, L.A.E. Intracellular water—An overlooked drug target? Cisplatin impact in cancer cells probed by neutrons. Phys. Chem. Chem. Phys. 2017, 19, 2702–2713. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.P.M.; Batista de Carvalho, A.L.M.; Mamede, A.P.; Santos, I.P.; Garcia Sakai, V.; Dopplapudi, A.; Cinque, G.; Wolna, M.; Gardner, P.; Batista de Carvalho, L.A.E. Chemotherapeutic Targets in Osteosarcoma: Insights from Synchrotron-MicroFTIR and Quasi-Elastic Neutron Scattering. J. Phys. Chem. B 2019, 123, 6968–6979. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Amado, A.M.; Parker, S.F.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Conformational insights and vibrational study of a promising anticancer agent: The role of the ligand in Pd(II)-amine complexes. New J. Chem. 2015, 39, 6274–6283. [Google Scholar] [CrossRef] [Green Version]
- Batista de Carvalho, A.L.M.; Parker, S.F.; Batista de Carvalho, L.A.E.; Marques, M.P.M. Novel platinum-based anticancer drug: A complete vibrational study. Acta. Crystallogr. C Struct. Chem. 2018, 74, 628–634. [Google Scholar] [CrossRef]
- Marques, M.P.M.; Batista de Carvalho, A.L.M.; Mamede, A.P.; Rudić, S.; Dopplapudi, A.; García Sakai, V.; Batista de Carvalho, L.A.E. Intracellular Water as a Mediator of Anticancer Drug Action. Int. Rev. Phys. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Fenimore, P.W.; Frauenfelder, H.; McMahon, B.H.; Parak, F.G. Slaving: Solvent Fluctuations Dominate Protein Dynamics and Functions. Proc. Natl. Acad. Sci. USA 2002, 99, 16047–16051. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.K.; Zhao, L.A.; Zewail, A.H. Water at DNA surfaces: Ultrafast dynamics in minor groove recognition. Proc. Natl. Acad. Sci. USA 2003, 100, 8113–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenimore, P.W.; Frauenfelder, H.; McMahon, B.H.; Young, R.D. Bulk-solvent and Hydration-shell Fluctuations, Similar to α- and β-fluctuations in Glasses, Control Protein Motions and Functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14408–14413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolov, A.P.; Roh, J.H.; Mamontov, E.; Sakai, V.G. Role of Hydration Water in Dynamics of Biological Macromolecules. Chem. Phys. 2008, 345, 212–218. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Chen, G.; Berendzen, J.; Fenimore, P.W.; Jansson, H.; McMahon, B.H.; Stroe, I.R.; Swenson, J.; Young, R.D. A Unified Model of Protein Dynamics. Proc. Natl. Acad. Sci. USA 2009, 106, 5129–5134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luby-Phelps, K. The Physical Chemistry of Cytoplasm and its Influence on Cell Function: An Update. Mol. Biol. Cell 2013, 24, 2593–2596. [Google Scholar] [CrossRef]
- Stadler, A.M.; Demmel, F.; Ollivier, J.; Seydel, T. Picosecond to nanosecond dynamics provide a source of conformational entropy for protein folding. Phys. Chem. Chem. Phys. 2016, 18, 21527–21538. [Google Scholar] [CrossRef] [Green Version]
- Bellissent-Funel, M.C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- Sokolov, A.P.; Grimm, H.; Kisliuk, A.; Dianoux, A.J. Slow relaxation process in DNA. J. Biol. Phys. 2001, 27, 313–327. [Google Scholar] [CrossRef]
- McDermott, M.L.; Vanselous, H.; Corcelli, S.A.; Petersen, P.B. DNA’s Chiral Spine of Hydration. ACS Central Sci. 2017, 3, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Michalarias, I.; Beta, I.A.; Li, J.C.; Ruffle, S.; Ford, R. The Interaction of Water with DNA—A Combined Inelastic Neutron Scattering and Infrared Spectroscopic Study. J. Mol. Liq. 2002, 101, 19–26. [Google Scholar] [CrossRef]
- Ruffle, S.V.; Michalarias, I.; Li, J.C.; Ford, R.C. Inelastic Incoherent Neutron Scattering Studies of Water Interacting with Biological Macromolecules. J. Am. Chem. Soc. 2002, 124, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Beta, I.A.; Michalarias, I.; Ford, R.C.; Li, J.C.; Bellissent-Funel, M.C. Quasi-elastic Neutron Scattering Study of Hydrated DNA. Chem. Phys. 2003, 292, 451–454. [Google Scholar] [CrossRef]
- Ford, R.C.; Ruffle, S.V.; Ramirez-Cuesta, A.J.; Michalarias, I.; Beta, I.; Miller, A.; Li, J.C. Inelastic Incoherent neutron Scattering Measurements of Intact Cells and Tissues and Detection of Interfacial Water. J. Am. Chem. Soc. 2004, 126, 4682–4688. [Google Scholar] [CrossRef] [PubMed]
- Michalarias, I.; Gao, X.L.; Ford, R.C.; Li, J.C. Recent progress on our understanding of water around biomolecules. J. Mol. Liq. 2005, 117, 107–116. [Google Scholar] [CrossRef]
- Wood, K.; Plazanet, M.; Gabel, F.; Kessler, B.; Oesterhel, D.; Tobias, D.J.; Zaccai, G.; Weik, M. Coupling of Protein and Hydration-Water Dynamics in Biological Membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 18049–18054. [Google Scholar] [CrossRef] [Green Version]
- Tehei, M.; Franzetti, B.; Wood, K.; Gabel, F.; Fabiani, E.; Jasnin, M.; Zamponi, M.; Oesterhelt, D.; Zaccai, G.; Ginzburg, M.; et al. Neutron Scattering Reveals Extremely Slow Cell Water in a Dead Sea Organism. Proc. Natl. Acad. Sci. USA 2007, 104, 766–771. [Google Scholar] [CrossRef] [Green Version]
- Jasnin, M.; Moulin, M.; Haertlein, M.; Zaccai, G.; Tehei, M. Down to Atomic-scale Intracellular Water Dynamics. EMBO Rep. 2008, 9, 543–547. [Google Scholar] [CrossRef]
- Sakai, V.G.; Arbe, A. Quasielastic neutron scattering in soft matter. Curr. Opin. Colloid. Interface Sci. 2009, 14, 381–390. [Google Scholar] [CrossRef]
- Mamontov, E.; Chu, X.Q. Water-Protein Dynamic Coupling and New Opportunities for Probing it at Low to Physiological Temperatures in Aqueous Solutions. Phys. Chem. Chem. Phys. 2012, 14, 11573–11588. [Google Scholar] [CrossRef]
- Schiro, G.; Fichou, Y.; Gallat, F.X.; Wood, K.; Gabel, F.; Moulin, M.; Hartlein, M.; Heyden, M.; Colletier, J.P.; Orecchini, A.; et al. Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins. Nat. Commun. 2015, 6, 6490. [Google Scholar] [CrossRef]
- Zaccai, G.; Bagyan, I.; Combet, J.; Cuello, G.J.; Deme, B.; Fichou, Y.; Gallat, F.X.; Josa, V.M.G.; von Gronau, S.; Haertlein, M.; et al. Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane. Sci. Rep. 2016, 6, 31434. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Araki, K.; Matsuo, T.; Yagi, H.; Yamada, T.; Shibata, K.; Mochizuki, H. Dynamical Behavior of Human alpha-Synuclein Studied by Quasielastic Neutron Scattering. PLoS ONE 2016, 11, e0151447. [Google Scholar] [CrossRef] [PubMed]
- Amann-Winkel, K.; Bellissent-Funel, M.C.; Bove, L.E.; Loerting, T.; Nilsson, A.; Paciaroni, A.; Schlesinger, D.; Skinner, L. X-ray and Neutron Scattering of Water. Chem. Rev. 2016, 116, 7570–7589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, S.F.; Lennon, D.; Albers, P.W. Vibrational Spectroscopy with Neutrons: A Review of New Directions. Appl. Spectrosc. 2011, 65, 1325–1341. [Google Scholar] [CrossRef]
- Parker, S.F.; Fernandez-Alonso, F.; Ramirez-Cuesta, A.J.; Tomkinson, J.; Rudić, S.; Pinna, R.S.; Gorini, G.; Castanon, J.F. Recent and future developments on TOSCA at ISIS. Dyn. Mol. Mater. II 2014, 554, 012003. [Google Scholar] [CrossRef]
- Pinna, R.S.; Rudić, S.; Capstick, M.J.; McPhail, D.J.; Pooley, D.E.; Howells, G.D.; Gorini, G.; Fernandez-Alonso, F. Detailed characterisation of the incident neutron beam on the TOSCA spectrometer. Nucl. Instrum. Meth. Phys. Res. A 2017, 870, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.; Dimeo, R.M.; Gehring, P.M.; Neumann, D.A. The high-flux backscattering spectrometer at the NIST Center for Neutron Research. Rev. Sci. Instrum. 2003, 74, 2759–2777. [Google Scholar] [CrossRef] [Green Version]
- Azuah, R.T.; Kneller, L.R.; Qiu, Y.M.; Tregenna-Piggott, P.L.W.; Brown, C.M.; Copley, J.R.D.; Dimeo, R.M. DAVE: A Comprehensive Software Suite for the Reduction, Visualization, and Analysis of Low Energy Neutron Spectroscopic Data. J. Res. Natl. Inst. Stand. Technol. 2009, 114, 341–358. [Google Scholar] [CrossRef]
- Wood, B.R. The importance of hydration and DNA conformation in interpreting infrared spectra of cells and tissues. Chem. Soc. Rev. 2016, 45, 1980–1998. [Google Scholar] [CrossRef]
- Paciaroni, A.; Orecchini, A.; Goracci, G.; Cornicchi, E.; Petrillo, C.; Sacchetti, F. Glassy character of DNA hydration water. J. Phys. Chem. B 2013, 117, 2026–2031. [Google Scholar] [CrossRef]
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Skoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instrum. Meth. A 2018, 896, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Li, J.C.; Ross, D.K. Evidence for 2 Kinds of Hydrogen-Bond in Ice. Nature 1993, 365, 327–329. [Google Scholar] [CrossRef]
- Li, J.C. Inelastic Neutron Scattering Studies of Hydrogen Bonding in Ices. J. Chem. Phys. 1996, 105, 6733–6755. [Google Scholar] [CrossRef]
- Chen, S.H.; Liu, L.; Chu, X.; Zhang, Y.; Fratini, E.; Baglioni, P.; Faraone, A.; Mamontov, E. Experimental evidence of fragile-to-strong dynamic crossover in DNA hydration water. J. Chem. Phys. 2006, 125, 171103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Pastor, N. The B- to A-DNA transition and the reorganization of solvent at the DNA surface. Biophys. J. 2005, 88, 3262–3275. [Google Scholar] [CrossRef] [Green Version]
- Franck, J.M.; Ding, Y.; Stone, K.; Qin, P.Z.; Han, S. Anomalously Rapid Hydration Water Diffusion Dynamics Near DNA Surfaces. J. Am. Chem. Soc. 2015, 137, 12013–12023. [Google Scholar] [CrossRef] [Green Version]
- Laage, D.; Hynes, J.T. A molecular jump mechanism of water reorientation. Science 2006, 311, 832–835. [Google Scholar] [CrossRef]
- Laage, D. Reinterpretation of the Liquid Water Quasi-Elastic Neutron Scattering Spectra Based on a Nondiffusive Jump Reorientation Mechanism. J. Phys. Chem. B 2009, 113, 2684–2687. [Google Scholar] [CrossRef]
- Drew, H.R.; Dickerson, R.E. Structure of a B-DNA Dodecamer. 3. Geometry of Hydration. J. Mol. Biol. 1981, 151, 535–556. [Google Scholar] [CrossRef]
- Dickerson, R.E.; Drew, H.R.; Conner, B.N.; Kopka, M.L.; Pjura, P.E. Helix geometry and hydration in A-DNA, B-DNA, and Z-DNA. Cold Spring Harb. Symp. Quant. Biol. 1983, 47, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Denisov, V.P.; Carlstrom, G.; Venu, K.; Halle, B. Kinetics of DNA hydration. J. Mol. Biol. 1997, 268, 118–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duboue-Dijon, E.; Fogarty, A.C.; Hynes, J.T.; Laage, D. Dynamical Disorder in the DNA Hydration Shell. J. Am. Chem. Soc. 2016, 138, 7610–7620. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol. Ther. 1987, 34, 155–166. [Google Scholar] [CrossRef]
- Sokolov, A.P.; Grimm, H.; Kahn, R. Glassy dynamics in DNA: Ruled by water of hydration? J. Chem. Phys. 1999, 110, 7053–7057. [Google Scholar] [CrossRef]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Bellissent-Funel, M.C.; Chen, S.H.; Dianoux, A.J. Experimental determination of the nature of diffusive motions of water molecules at low temperatures. Phys. Rev. A 1985, 31, 1913–1917. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.P.M.; Batista de Carvalho, L.A.E.; Batista de Carvalho, A.L.M.; Rudić, S. Probing Drug Pharmacodynamics by INS—The Effect of Cisplatin-Like Anticancer Drugs on DNA. 2018. Available online: https://data.isis.stfc.ac.uk/doi/STUDY/103197359/ (accessed on 19 November 2019).









| Sample | Backbone | H2O/Spine Hydration | H2O/Hydration Shell a | Fast Localised Motions a | ||
|---|---|---|---|---|---|---|
| DT (×10−5 cm2 s−1) | τT (ns) | τT (ns) | DT (×10−5 cm2 s−1) | τT (ps) | τT (ps) | |
| D2O-DNAhyd | 1.0621 ± 0.0291 | 0.6607 ± 0.0221 | _ | _ | _ | _ |
| D2O-DNAhyd + cisplatin | 0.5532 ± 0.0322 | 0.8557 ± 0.0784 | _ | _ | _ | _ |
| D2O-DNAhyd + Pd2Spm | 1.2267 ± 0.0962 | 0.4796 ± 0.0594 | _ | _ | _ | _ |
| H2O-DNAhyd | 1.0621 ± 0.0291 | 0.6607 ± 0.0221 | 0.1995 ± 0.0223 | 0.723 ± 0.002 | 10.13 ± 0.32 | 3.591 ± 0.006 |
| H2O-DNAhyd + cisplatin | 0.5532 ± 0.0322 | 0.8557 ± 0.0784 | 0.1550 ± 0.0173 | 0.901 ± 0.004 | 7.40 ± 0.36 | 3.270 ± 0.003 |
| H2O-DNAhyd + Pd2Spm | 1.2267 ± 0.0962 | 0.4796 ± 0.0594 | 0.2067 ± 0.0112 | 0.744 ± 0.004 | 8.38 ± 0.86 | 3.387 ± 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, M.P.M.; Batista de Carvalho, A.L.M.; Mamede, A.P.; Dopplapudi, A.; Rudić, S.; Tyagi, M.; Garcia Sakai, V.; Batista de Carvalho, L.A.E. A New Look into the Mode of Action of Metal-Based Anticancer Drugs. Molecules 2020, 25, 246. https://doi.org/10.3390/molecules25020246
Marques MPM, Batista de Carvalho ALM, Mamede AP, Dopplapudi A, Rudić S, Tyagi M, Garcia Sakai V, Batista de Carvalho LAE. A New Look into the Mode of Action of Metal-Based Anticancer Drugs. Molecules. 2020; 25(2):246. https://doi.org/10.3390/molecules25020246
Chicago/Turabian StyleMarques, M. Paula M., Ana L. M. Batista de Carvalho, Adriana P. Mamede, Asha Dopplapudi, Svemir Rudić, Madhusudan Tyagi, Victoria Garcia Sakai, and Luís A. E. Batista de Carvalho. 2020. "A New Look into the Mode of Action of Metal-Based Anticancer Drugs" Molecules 25, no. 2: 246. https://doi.org/10.3390/molecules25020246
APA StyleMarques, M. P. M., Batista de Carvalho, A. L. M., Mamede, A. P., Dopplapudi, A., Rudić, S., Tyagi, M., Garcia Sakai, V., & Batista de Carvalho, L. A. E. (2020). A New Look into the Mode of Action of Metal-Based Anticancer Drugs. Molecules, 25(2), 246. https://doi.org/10.3390/molecules25020246