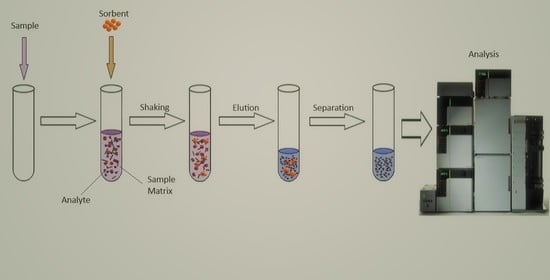
Recent Materials Developed for Dispersive Solid Phase Extraction
Abstract
:1. Introduction
2. Sorbents Based on Silica
3. Sorbents Based on Magnetic Nanoparticles
4. Sorbents Based on Molecularly Imprinted Polymers
5. Sorbents Based on Carbon
5.1. Graphite and Graphene
5.2. Carbon Nanotubes
| Material | Target Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (ng L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| AMM-AC | Cu | food | 0.8–180 | 310 | 95.7–103.6 | FAAS | [38] |
| AC-PAN | antibiotics | waste | - | 50–200 | 98–103 | HPLC-DAD | [35] |
| ACF | pesticides | food | - | 1.14–5.89 b | 70–120 | GC-ECD, GC-MS/MS | [53] |
| GO@SiO2 | phytohormones | food | 0.5–50 | 30,000–50,000 | 98.1–118.4 | HPLC-UVD | [39] |
| GO@SiO2 | melatonin, tryptophan | food | 0.25–500 | 50,000–100,000 | 89.1–114.8 | HPLC-DAD, UPLC-MS/MS | [36] |
| 3D-Mag-CMGO | disperse dyes | waste | 5–1000 | 500–2480 | 70–109 | UFLC-MS/MS | [41] |
| 3D-Mag-CMGO | disperse dyes | environmental | 0.005–5 | 0.17–10.2 | 80.0–112 | UFLC-MS/MS | [42] |
| 3D-Mag-CMGO | pharmaceuticals | environmental | 0.001–0.5 | 0.034–0.63 | 78.0–109 | UFLC-MS/MS | [43] |
| tri-BuA-rGO | pesticides | food | 1–500 b | 0.33–16.5 b | 72.1–120.5 | UHPLC-MS/MS, GC-MS/MS | [40] |
| rGO-CHNPs | velpatasvir | biological | 0.5–45 | 40 | 97.96–103.0 | CPE-FLD | [44] |
| G/Sep | ryboflavin | food | 80–700 | 3000 | 95–104 | FLD | [54] |
| G/Sep | PAH | waste | 0.39–45 | 96–830 | 95.2–100.2 | HPLC-FLD | [37] |
| MCN | sulfonamides | environmental | 0.09–200 | 20–50 | 82.3–110.5 | HPLC-DAD | [45] |
| V-g-C3N4 | PCA | environmental | - | 0.5–2 | 80.12–119.17 | DART-MS, HPLC-UVD | [46] |
| MWCNTs | PAH, Cd, Cr, Pb | environmental | 0.01–50 | 3–30 | 80.7–116.1 | GC-MS/AAS | [4] |
| MWCNTs | PAH | environmental | - | - | - | GC-MS | [47] |
| MWCNTs | pharmaceuticals | environmental | 0.02–2.5 | 1–8 | 85.99–120.05 | LC-MS/MS | [48] |
| MWCNTs | β-blockers | environmental | 0.005–0.5 | 1 | 80.2–135.7 | GC-MS, LC-MS/MS | [49] |
| QA-Mag-CCNTs | perchlorate | biological | 0.01–1 b | 0.00249 b | 85.2–107 | UFLC-MS/MS | [55] |
| PEG-CNT-MNP | Z-ligustilide | herbal | - | - | 98.9 | HPLC-DAD, HPLC-MS/MS | [56] |
| M-BG | Cr | environmental | 0.4–40 | 100 | 94.4–106 | FO-LADS | [51] |
| DES-MBG | pesticides | environmental | 0.0002–2 | 0.03–0.27 | 80–119 | GC-MECD | [52] |
| OH-MMWCNTs @CTAB | PAH | engine exhaust | 0.02–1 | 9–100 | 72.65–96.54 | GC-MS | [50] |
6. Sorbents Based on Layered Double Hydroxides
| Material | Target Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (ng L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| BCS-LDH | Fe | food, environmental | 0.5–100 | 400 | 99.04–102.3 | UV/Vis | [59] |
| Mg-Al-AHDNA-LDH | Cd, Co, Cr, Ni, Pb | biological | 2–725 | 600–2400 | 95–102 | FAAS | [61] |
| LDH-APDC | Cr | biological | 8–640 | 2400 | 96–101 | FAAS | [57] |
| LDH-ALA | Cr | environmental | 20–700 | 7100 | 97.67–110.08 | FAAS | [58] |
| DAMP-CuNCs | Cr | environmental | 116–812 | 36,000 | 101.6–106.9 | FLD | [60] |
7. Sorbents Based on Metallic Organic Frameworks
8. Sorbents Based on Porous Polymers
9. Other Novel Sorbents of Note
10. Summary and Conclusions
Funding
Conflicts of Interest
References
- Majors, R.E. Sample Preparation Fundamentals for Chromatography; Agilent Technologies: Mississauga, ON, Canada, 2013. [Google Scholar]
- Faraji, M.; Yamini, Y.; Gholami, M. Recent Advances and Trends in Applications of Solid-Phase Extraction Techniques in Food and Environmental Analysis. Chromatographia 2019, 82, 1207–1249. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Caban, M.; Bielicka-Giełdoń, A.; Stepnowski, P. Optimization of a procedure for the simultaneous extraction of polycyclic aromatic hydrocarbons and metal ions by functionalized and non-functionalized carbon nanotubes as effective sorbents. Talanta 2017, 165, 405–411. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Han, D.; Lei, Y.; Zhu, T. Dispersion solid-phase extraction of flavonoid with amphilic monomers, N-vinyl pyrrolidone and 1H,1H,7H-dodecafluoroheptyl methacrylate based poly(styrene-divinylbenzene) and silica. Anal. Methods 2018, 10, 4680–4688. [Google Scholar] [CrossRef]
- Lawal, A.; Wong, R.C.S.; Tan, G.H.; Abdulra’uf, L.B.; Alsharif, A.M.A. Recent Modifications and Validation of QuEChERS-dSPE Coupled to GC-MS and LC-MS Instruments for Determination of Pesticide/Agrochemical Residues in Fruits and Vegetables: Review. J. Chromatogr. Sci. 2018, 56, 565–669. [Google Scholar] [CrossRef] [Green Version]
- Si, R.; Han, Y.; Wu, D.; Qiao, F.; Bai, L.; Wang, Z.; Yan, H. Ionic liquid-organic-functionalized ordered mesoporous silica-integrated dispersive solid-phase extraction for determination of plant growth regulators in fresh Panax Ginseng. Talanta 2020, 207, 120247. [Google Scholar] [CrossRef]
- Casado, N.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I. Bi-functionalised mesostructured silicas as reversed phase/strong anion exchange sorbents. Application to extraction of polyphenols prior to their quantification by UHPLC with ion-trap mass spectrometry detection. Microchim. Acta 2019, 186, 164. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shiri, F.; Kordestani, D.; Mohammadi, P.; Alizadeh, A. SBA-15/Metforminas a novel sorbent combined with surfactant-assisted dispersive liquid-liquid microextraction (SA-DLLME) for highly sensitive determination of Pb, Cd and Ni in food and environmental samples. J. Iran. Chem. Soc. 2018, 15, 753–768. [Google Scholar] [CrossRef]
- Qin, P.; Yang, Y.; Li, W.; Zhang, J.; Zhou, Q.; Lu, M. Amino-functionalised mesoporous silica nanospheres (MSN-NH2) as sorbent for extraction and concentration of synthetic dyes from foodstuffs prior to HPLC analysis. Anal. Methods 2019, 11, 105–112. [Google Scholar] [CrossRef]
- Artiushenko, O.; Ávila, E.P.; Nazarovsky, M.; Zaitsev, V. Reusable hydroxamate immobilized silica adsorbent for dispersive solid phase extraction and separation of rare earth metal ions. Sep. Purif. Technol. 2020, 231, 115934. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Q.; Chen, C.; Li, X.; Qing, G.; Sun, T.; Liang, X. Smart polymers driven by multiple and tunable hydrogen bonds for intact phosphoprotein enrichment. Sci. Technol. Adv. Mater. 2019, 20, 858–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, P.H.; Lin, S.L.; Fuh, M.R. Poly(lauryl methacrylate-co-1,6-hexanediol ethoxylate diacrylate) modified silica-based dispersive solid-phase extraction for determination of phenylurea herbicides in environmental water samples. Int. J. Environ. Anal. Chem. 2018, 98, 830–843. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, F.; Ma, X.; Yue, M.; Li, Y.; Liu, J.; You, J. Quaternary ammonium-functionalized MCM-48 mesoporous silica as a sorbent for the dispersive solid-phase extraction of endocrine disrupting compounds in water. J. Chromatogr. A 2018, 1557, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Câmara, J.S.; Sierra, I. Dispersive Solid-Phase Extraction of Polyphenols from Juice and Smoothie Samples Using Hybrid Mesostructured Silica Followed by Ultra-high-Performance Liquid Chromatography-Ion-Trap Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 955–967. [Google Scholar] [CrossRef]
- Sadeghi, M.; Rostami, E.; Kordestani, D.; Veisi, H.; Shamsipur, M. Simultaneous determination of ultra-low traces of lead and cadmium in food and environmental samples using dispersive solid-phase extraction (DSPE) combined with ultrasound-assisted emulsification microextraction based on the solidification of floating organic drop (UAEME-SFO) followed by GFAAS. RSC Adv. 2017, 7, 27656–27667. [Google Scholar]
- Pashaei, Y.; Ghorbani-Bidkorbeh, F.; Sherkachi, M. Superparamagnetic graphite oxide-based dispersive-solid phase extraction for preconcentration and determination of tamsulosin hydrochloride in human plasma by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2017, 1499, 21–29. [Google Scholar] [CrossRef]
- Qi, X.; Gao, S.; Ding, G.; Tang, A.N. Synthesis of surface Cr (VI)-imprinted magnetic nanoparticles for selective dispersive solid-phase extraction and determination of Cr (VI) in water samples. Talanta 2017, 162, 345–353. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.A. Core-shell poly(dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LC-MS analysis. Food Chem. 2017, 215, 362–368. [Google Scholar] [CrossRef]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.A. Determination of phthalic acid esters in water samples using core-shell poly(dopamine) magnetic nanoparticles and gas chromatography tandem spectrometry. J. Chromatogr. A 2017, 1530, 35–44. [Google Scholar] [CrossRef]
- Jiménez-Skrzypek, G.; González-Sálamo, J.; Valera-Martínez, D.A.; González-Curbelo, M.A.; Hernández-Borges, J. Analysis of phthalic acid esters in sea water and sea sand using polymer-coated magnetic nanoparticles as extraction sorbent. J. Chromatogr. A 2020, 1611, 460620. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, A.; Islam, S.S. Magnetic Fe3O4@poly(methacrylic acid) particles for selective preconcentration of trace arsenic species. Microchim. Acta 2017, 184, 2007–2014. [Google Scholar] [CrossRef]
- Hajilari, F.; Farhadi, K.; Eskandari, H. Extraction and Trace Analysis of Trihalomethanes in Water Samples Using Zein@Fe3O4 Nanocomposite. Bull. Environ. Cotnam. Toxicol. 2019, 102, 581–588. [Google Scholar] [CrossRef]
- Li, D.; Li, T.; Wang, L.; Ji, S. A polyvinyl alcohol-coated core-shell magnetic nanoparticle for the extraction of aminoglycoside antibiotics residues from honey samples. J. Chromatogr. A 2018, 1581–1582, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zaman, B.T.; Erulaş, A.F.; Chormey, D.S.; Bakirdere, S. Combination of stearic acid coated magnetic nanoparticle based sonication assisted dispersive solid phase extraction and slotted quartz tube-flame atomic absorption spectrophotometry for the accurate and sensitive determination of lead in red pepper sample and assessment of green profile. Food Chem. 2020, 303, 125396. [Google Scholar] [PubMed]
- Akkaya, E.; Bozyiğit, G.D.; Bakirdere, S. Simultaneous determination of 4-tert-octylphenol, chlorpyrifos-ethyl and penconazole by GC-MS after sensitive and selective preconcentration with stearic acid coated magnetic nanoparticles. Microchem. J. 2019, 146, 1190–1194. [Google Scholar] [CrossRef]
- Azizi, A.; Shahhoseini, F.; Bottaro, C.S. Magnetic molecularly imprinted polymers prepared by reversible addition fragmentation chain transfer polymerization for dispersive solid phase extraction of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2020, 1610, 460534. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Wang, J.; Li, L.; Li, R. Hydrophilic molecularly imprinted dispersive solid-phase extraction coupled with liquid chromatography for determination of azoxystrobin residues in cucumber. Iran. Polym. J. 2019, 28, 725–734. [Google Scholar] [CrossRef]
- Kavakandi, M.G.; Behbahani, M.; Omidi, F.; Hesam, G. Application of Ultrasonic Assisted-Dispersive Solid Phase Extraction Based on Ion-Imprinted Polymer Nanoparticles for Preconcentration and Trace Determination of Lead Ions in Food and Water Samples. Food Anal. Methods 2017, 10, 2454–2466. [Google Scholar] [CrossRef]
- Behbahani, M.; Omidi, F.; Kakavandi, M.G.; Hesam, G. Selective and sensitive determination of silver ions at trace levels based on ultrasonic-assisted dispersive solid-phase extraction using ion-imprinted polymer nanoparticles. Appl. Organomet. Chem. 2017, 31, e3758. [Google Scholar] [CrossRef]
- Chen, X.; Ye, N. A graphene oxide surface-molecularly imprinted polymer as a dispersive solid-phase extraction adsorbent for the determination of cefadroxil in water samples. RCS Adv. 2017, 7, 34077–34085. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Liu, J.; Li, J.; Wang, X.; Lv, M.; Cui, R.; Chen, L. Dual-template molecularly imprinted polymers for dispersive solid-phase extraction of fluoroquinolones in water samples coupled with high performance liquid chromatography. Analyst 2019, 144, 1292–1302. [Google Scholar] [CrossRef]
- Luo, J.; Chen, N.; Yang, Z.; Han, J.; Zhu, W.; Hong, J.; Zhou, X. Determination of active ingredients in Chinese medicine Danning Tablets using dispersion solid-phase extraction by molecular imprinting nanomaterials coupled with HPLC-DAD. Anal. Methods 2017, 9, 2585–2589. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Nomngongo, P.N. Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J. Pharm. Anal. 2019, 9, 117–126. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, X.; Qin, P.; Yang, Y.; Tian, S.; Yang, H.; Lu, M. Simultaneous Determination of Melatonin, L-Tryptophan, and two L-Tryptophan-Derived Esters in Food by HPLC with Graphene Oxide-SiO2 Nanocomposite as the Adsorbent. Food Anal. Methods 2018, 11, 2438–2446. [Google Scholar] [CrossRef]
- Mateos, R.; Vera-López, S.; Saz, M.; Díez-Pascual, A.M.; San Andrés, M.P. Graphene/sepiolite mixtures as dispersive solid-phase extraction sorbents for the analysis of polycyclic aromatic hydrocarbons in wastewater using surfactant aqueous solutions for desorption. J. Chromatogr. A 2019, 1596, 30–40. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Mohammadiazar, S.; Ardalan, S. New modified carbon based solid phase extraction sorbent prepared form wild cherry stone as natural raw material for pre-concentration and determination of trace amounts of copper in food samples. Microchem. J. 2019, 147, 666–673. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Zhang, X.; Xiao, R.; Lu, M.; Cai, Z. Graphene oxide-SiO2 nanocomposite as the adsorbent for extraction and preconcentration of plant hormones for HPLC analysis. J. Chromatogr. B 2017, 1046, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, M.; Zhu, L.; Chen, H.; Liu, X.; Lu, C. Facile synthesis of amine-functional reduced graphene oxides as modified quick, easy, cheap, effective, rugged and safe adsorbent for multi-pesticide residues analysis of tea. J. Chromatogr. A 2018, 1531, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.L.; Liu, J.N.; Shi, L.L.; Chen, G.S. Determination of Nine Sensitizing Disperse Dyes in Dyeing Wastewater by Solid Phase Extraction-Liquid Chromatography-Mass Spectrometry. Chin. J. Anal. Chem. 2012, 39, 39–44. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Li, X.P.; Yao, S.S.; Zhan, P.P.; Liu, J.C.; Xu, C.P.; Lu, Y.Y.; Chen, X.H.; Jin, M.C. Fast throughput determination of 21 allergenic disperse dyes from river water using reusable three-dimensional interconnected magnetic chemically modified graphene oxide followed by liquid chromatography-tandem quadrupole mass spectrometry. J. Chromatogr. A 2016, 1431, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Zhao, Y.G.; Qiu, Q.L.; Zhu, Y.; Min, J.Q.; Jin, M.C. A fast and high throughput LC-MS/MS method for the determination of 58 human and veterinary drugs in river water. Anal. Methods 2017, 9, 4228–4233. [Google Scholar] [CrossRef]
- El-Wekil, M.M.; Ali, H.R.H.; Marzouk, A.A.; Ali, R. Enhanced dispersive solid phase extraction assisted by cloud point strategy prior to fluorometric determination of anti-hepatitis C drug velpatasvir in pharmaceutical tablets and body fluids. RSC Adv. 2018, 8, 13292–13300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, W.; Zhu, W.; Yang, Y.; Qin, P.; Zhu, Q.; Lu, M.; Cai, Z. Mesoporous graphitic carbon nitride as an efficient sorbent for extraction of sulfonamides prior to HPLC analysis. Microchim. Acta 2019, 186, 279. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Si, L.; Du, Q.; Li, H.; Bi, W.; Chen, D.D.Y. High throughput screening of phenoxy carboxylic acids with dispersive solid phase extraction followed by direct analysis in real time mass spectrometry. Anal. Chim. Acta 2017, 996, 20–28. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Sikorska, M.; Leszczyńska, D.; Stepnowski, P. Helical Multi-walled Carbon Nanotubes as an efficient material for the Dispersive Solid-Phase Extraction of Low and High Molecular Weight Polycyclic Aromatic Hydrocarbons from Water Samples: Theoretical Study. Water Air Soil Pollut. 2018, 229, 253. [Google Scholar] [CrossRef] [Green Version]
- Jakubus, A.; Godlewska, K.; Gromelski, M.; Jagiello, K.; Puzyn, T.; Stepnowski, P.; Paszkiewicz, M. The possibility to use multi-walled carbon nanotubes as sorbent for dispersive solid phase extraction of selected pharmaceuticals and their metabolites: Effect of extraction condition. Microchem. J. 2019, 146, 1113–1125. [Google Scholar] [CrossRef]
- Jakubus, A.; Gromelski, M.; Jagiello, K.; Puzyn, T.; Stepnowski, P.; Paszkiewicz, M. Dispersive solid-phase extraction using multi-walled carbon nanotubes combined with liquid chromatography-mass spectrometry for the analysis of β-blockers: Experimental and theoretical studies. Microchem. J. 2019, 146, 258–269. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Zhang, X.; Li, D. Preparation of micelle supported magnetic hydroxylated multi-walled carbon nanotubes based DSPE for determination of PAHs. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012003. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, S.M.; Shemirani, F. Carbon nanotube-based magnetic bucky gels in developing solid-phase extraction: Application in rapid speciation analysis of Cr (VI) and Cr (III) in water samples. Int. J. Environ. Anal. Chem. 2017, 97, 1065–1079. [Google Scholar] [CrossRef]
- Yousefi, S.M.; Shemirani, F.; Ghorbanian, S.A. Deep eutectic solvent magnetic bucky gels in developing solid phase extraction: Application for ultra trace analysis of organochlorine pesticides by GC-micro ECD using a large-volume injection technique. Talanta 2017, 168, 73–81. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, A.; Singh, S.P. Inexpensive, effective novel activated carbon fibers for sample cleanup: Application to multipesticide residue analysis in food commodities using QuEChERS method. Anal. Bioanal. Chem. 2018, 410, 2241–2251. [Google Scholar] [CrossRef]
- Mateos, R.; Vera-López, S.; Díez-Pascual, A.M.; San Andrés, M.P. Dispersive solid phase extraction/fluorescence analysis of riboflavin using sepiolite as sorbent. Appl. Clay Sci. 2018, 163, 279–290. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, Y.; Wang, F.L.; Zhou, J.; Zhao, Q.M.; Zeng, X.Q.; Hu, M.Q.; Jin, M.C.; Zhu, Y. Determination of perchlorate from tea leaves using quaternary ammonium modified magnetic carboxyl-carbon nanotubes followed by liquid chromatography-tandem quadrupole mass spectrometry. Talanta 2018, 185, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Liu, Y.M.; Jia, Y.W.; Wan, L.H.; Liao, X. PEGylation of magnetic multi-walled carbon nanotubes for enhanced selectivity of dispersive solid phase extraction. Mat. Sci. Eng. C 2017, 71, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Barfi, B.; Asghari, A.; Rajabi, M. Toward use of a nano layered double hydroxide/ammonium pyrrolidine dithiocarbamate in speciation analysis: One-step dispersive solid-phase extraction of chromium species in human biological samples. Arab. J. Chem. 2017, 13, 568–579. [Google Scholar] [CrossRef]
- Dos Santos Azevedo Leite, V.; de Jesus, B.G.L.; de Oliveira Duarte, V.G.; Constantino, V.R.L.; Izumi, C.M.S.; Tronto, J.; Pinto, F.G. Determination of chromium (VI) by dispersive solid-phase extraction using dissolvable Zn-Al layered double hydroxide intercalated with l-Alanine as adsorbent. Microchem. J. 2019, 146, 650–657. [Google Scholar] [CrossRef]
- Hami, Y.; Fat’hi, R.M. A colorimetric-dispersive solid-phase extraction method for the sensitive and selective determination of iron using dissolvable bathocuproinedisulfonic acid-intercalated layered double hydroxide nanosheet. New J. Chem. 2018, 42, 5489–5498. [Google Scholar] [CrossRef]
- Khonkayan, K.; Sansuk, S.; Srijaranai, S.; Tuntulani, T.; Saiyasombat, C.; Busayaporn, W.; Ngeontae, W. New approach for detection of chromate ion by preconcentration with mixed metal hydroxide coupled with fluorescence sensing of copper nanoclusters. Microchim. Acta 2017, 184, 2965–2974. [Google Scholar] [CrossRef]
- Rajabi, M.; Arghavani-Beydokhti, S.; Barfi, B.; Asghari, A. Dissolvable layered double hydroxide as an efficient nanosorbent for centrifugeless air agitated dispersive solid-phase extraction for potentially toxic metal ions from bio-fluid samples. Anal. Chim. Acta 2017, 957, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Xu, X.; Zhang, L. Metal-organic framework-derived three-dimensional porous graphitic octahedron carbon cages-encapsulated copper nanoparticles hybrids as highly efficient enrichment material for simultaneous determination of four fluoroquinolones. J. Chromatogr. A 2018, 1533, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Taghvimi, A.; Tabrizi, A.B.; Dastmalchi, S.; Javadzadeh, Y. Metal organic framework based carbon porous as an efficient dispersive solid phase extraction adsorbent for analysis of methamphetamine from urine matrix. J. Chromatogr. B 2019, 1109, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Tayebee, R.; Abdar, A.; Sani, F.N. Synthesis of zinc-based metal-organic framework with histamine as an organic linker for the dispersive solid-phase extraction of organophosphorus pesticides in water and fruit juice samples. J. Chromatogr. A 2019, 1597, 39–45. [Google Scholar] [CrossRef]
- Ghani, M. A dissolvable hierarchical layered double hydroxide templated from porous zeolitic imidazolate framework-67 for dispersive solid-phase extraction of bisphenol A. Anal. Methods 2019, 11, 4184–4189. [Google Scholar] [CrossRef]
- Gao, M.; Liu, W.; Wang, X.; Li, Y.; Zhou, P.; Shi, L.; Ye, B.; Dahlgren, R.A.; Wang, X. Hydrogen-bonding-induced efficient dispersive solid phase extraction of bisphenols and their derivatives in environmental waters using surface amino-functionalized MIL-101(Fe). Microchem. J. 2019, 145, 1151–1161. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Zhang, C.; Shao, Y.; She, Y.; Jin, F.; Jin, M.; et al. Metal-organic framework UiO-66 for rapid dispersive solid phase extraction of neonicotinoid insecticides in water samples. J. Chromatogr. B 2018, 1077–1078, 92–97. [Google Scholar] [CrossRef]
- Peña-Méndez, E.M.; Mawale, R.M.; Conde-González, J.E.; Socas-Rodríguez, B.; Havel, J.; Ruiz-Pérez, C. Metal organic framework composite, nano-Fe3O4@Fe(benzene-1,3,5-tricarboxylic acid) for solid phase extraction of blood lipid regulators from water. Talanta 2020, 207, 120275. [Google Scholar]
- Lv, Z.; Sun, Z.; Song, C.; Lu, S.; Chen, G.; You, J. Sensitive and background-free determination of thiols from wastewater samples by MOF-5 extraction coupled with high-performance liquid chromatography with fluorescence detection using a novel fluorescence probe of carbazole-9-ethyl-2-maleimide. Talanta 2016, 161, 228–237. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Marć, M.; Szczepańska, N.; Namieśnik, J. New Polymeric Materials for Solid Phase Extraction. Crit. Rev. Anal. Chem. 2017, 47, 373–383. [Google Scholar]
- Oellig, C.; Shmid, S. Polyethyleneimine as weak anionic exchanger adsorbent for clean-up in pesticide residue analysis of fruits and vegetables. J. Chromatogr. A 2019, 1597, 9–17. [Google Scholar] [CrossRef]
- Arnnok, P.; Patdhanagul, N.; Burakham, R. Dispersive solid-phase extraction using polyaniline-modified zeolite NaY as a new sorbent for multiresidue analysis of pesticides in food and environmental samples. Talanta 2017, 164, 651–661. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Wu, Q.; Yang, L.; Xie, T. Sensitive Simultaneous Determination of Synthetic Food Colorants in Preserved Food Samples by Capillary Electrophoresis with Contactless Conductivity Detection. Food Anal. Methods 2018, 11, 1608–1618. [Google Scholar]
- Zhang, J.; Liu, D.; Shi, Y.; Sun, C.; Niu, M.; Wang, R.; Hu, F.; Xiao, D.; He, H. Determination of quinolones in wastewater by porous β-cyclodextrin polymer based solid-phase extraction coupled with HPLC. J. Chromatogr. B 2017, 1068–1069, 24–32. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wang, X.; Ming, M.; Li, J.; Ye, N. Determination of sulfonamides in milk by capillary electrophoresis with PEG@MoS2 as a dispersive solid-phase extraction sorbent. R. Soc. Open Sci. 2018, 5, 172104. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Wang, X.; Ye, N. Molybdenum Disulfide as a Dispersive Solid-Phase Extraction Adsorbent for Determination of Sulfonamide Residues in Water Samples Using Capillary Electrophoresis. ChemistrySelect 2017, 2, 9046–9051. [Google Scholar] [CrossRef]
- Dong, S.; Lou, Q.; Huang, G.; Guo, J.; Wang, X.; Huang, T. Dispersive solid-phase extraction based on MoS2/carbon dot composite combined with HPLC to determine brominated flame retardants in water. Anal. Bioanal. Chem. 2018, 410, 7337–7346. [Google Scholar] [CrossRef]
- Chu, J.M.; Qi, C.B.; Huang, Y.Q.; Jiang, H.P.; Hao, Y.H.; Yuan, B.F.; Feng, Y.Q. Metal Oxide-Based Selective Enrichment Combined with Stable Isotope Labeling-Mass Spectrometry Analysis for Profiling of Ribose Conjugates. Anal. Chem. 2015, 87, 7364–7372. [Google Scholar] [CrossRef]
- Chu, J.M.; Yin, T.L.; Zheng, S.J.; Yang, J.; Yuan, B.F.; Feng, Y.Q. Metal oxide-based dispersive solid-phase extraction coupled with mass spectrometry analysis for determination of ribose conjugates in human follicular fluid. Talanta 2017, 167, 506–512. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Huang, X.; Jia, C.; Cao, Y.; Hu, L.; Lu, R.; Zhang, S.; Gao, H.; Zhou, W.; et al. Ionic liquid-modified luffa sponge fibers for dispersive solid-phase extraction of benzylurea insecticides from water and tea beverage samples. New J. Chem. 2018, 420, 8791–8799. [Google Scholar] [CrossRef]
- Yang, X.; Lin, X.W.; Mi, Y.D.; Gao, H.; Li, J.; Zhang, S.; Zhou, W.; Lu, R. Ionic liquid-type surfactant modified attapulgite as a novel and efficient dispersive solid phase material for fast determination of pyrethroids in tea drinks. J. Chromatogr. B 2018, 1089, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Fu, Q.; Wang, M.; Zhang, K.; Zeng, J.; Wang, L.; Xia, Z.; Gao, D. Facile synthesis of porous covalent organic frameworks for the effective extraction of nitroaromatic compounds from water samples. Anal. Chim. Acta 2019, 1084, 21–23. [Google Scholar] [CrossRef]
- Wu, F.F.; Chen, Q.Y.; Ma, X.J.; Li, T.T.; Wang, L.F.; Hong, J.; Sheng, Y.H.; Ye, M.L.; Zhu, Y. N-doped magnetic covalent organic frameworks for preconcentration of allergenic disperse dyes in textiles of fall protection equipment. Anal. Methods 2019, 11, 3381–3387. [Google Scholar] [CrossRef]

| Material | Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (ng L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| SiO2-BPHA | rare earth elements | aqueous solution | - | - | - | ICP-OES | [12] |
| NIPAAm-co-ABTA0.35 @SiO2 | α-casein | food | - | - | - | HPLC-DAD | [13] |
| LMA-HEDA@SiO2 | herbicides | environmental | 0.1–4 | 27–53 | 80.1–97.9 | HPLC-UVD | [14] |
| QTA-MCM-48 | EDC | environmental | 0.005–0.5 | 1.2–2.6 | 95.4–104 | HPLC-FLD | [15] |
| HMS-RPC8-SAX2 | polyphenols | food | - | 1–560 | 70–101 | UHPLC-MS/MS | [9] |
| HMS-C18 | polyphenols | food | 0.02–100 | 10–50 | 48–103 | UHPLC-MS/MS | [16] |
| SBA-15/Met | Cd, Ni, Pb | food, environmental | 0.0025–10 | 1–2 | 97.9–101.5 | GFAAS | [10] |
| SBA-15/CCMet | Cd, Pb | food, environmental | 0.001–15 | 0.2, 0.5 | 96.4–101.9 | GFAAS | [17] |
| MSN-NH2 | synthetic dyes | food | 0.45–1000 | 0.10, 0.30 | 80.0–116.8 | HPLC-DAD | [11] |
| IL-WFOMS | plant growth regulators | herbal | 0.05–22.5 b | 0.003–0.008 b | 77.6–98.3 | HPLC-FLD | [8] |
| Material | Target Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (ng L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| GO-Fe3O4 | tamsulosin hydrochloride | biological | 0.5–50 | 170 | 98.0–101.4 | HPLC-UVD | [18] |
| Fe3O4@pDA | mycotoxins | food | 20–400 | 290–4800, 0.41–5.82 b | 77–120 | HPLC-MS/MS | [20] |
| Fe3O4@pDA | phthalic acid esters | environmental | 0.5–500 | 9–20 c | 71–120 | GC-MS/MS | [21] |
| Fe3O4@pDA | phthalic acid esters | environmental | 0.1–250 | 1.38–3.19 c 0.020–4.0 b c | 70–120 | GC-MS/MS | [22] |
| M-PMA | As | environmental | 0–100 | 2.98–9.95 | 99–102 | HG-MP-AES | [23] |
| Zein@Fe3O4 | trihalomethanes | environmental | 0.5–100 | 100–360 | 96.68–101.2 | GC-MECD | [24] |
| Fe3O4@PVA | antibiotics | food | 20–4000 b | 0.913–1.23 b | 82.9–100.7 | HILIC-MS/MS | [25] |
| SAC-MNP | Pb | food | 30–250 | 10,000 | 102.6–106.6 | SQT-FAAS | [26] |
| SAC-MNP | EDC | environmental | 1–1000 | 0.28–10,000 | 95.3–107.8 | GC-MS | [27] |
| Material | Target Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (ng L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4@Cr(VI)IIPs | Cr | environmental | 4–140 | 29,000 | 96.1–99.2 | FAAS | [19] |
| Pb-IIP | Pb | food | 3–900 | 700 | 96.0–104.0 | FAAS | [30] |
| Ag-IIP | Ag | environmental | 0.5–600 | 90 | 96.2–105.7 | FAAS | [31] |
| MMIP | PAH | environmental | 0.002–50 | 1–100 | 4.5–97 | GC-MS/MS | [28] |
| GO-MIP | cefadroxil | environmental | 40–6000 | 10,000 | 72.5–104.8 | UPLC-DAD | [32] |
| dt-MIP | fluoroquinolones | environmental | 1–200 | 220, 360 | 80.9–101.0 | HPLC-DAD | [33] |
| HMIM | azoxystrobin | food | 100–10,000 b | 0.324 b | 85.93–88.89 | HPLC-UVD | [29] |
| PD-MMIP | PD, resveratrol | medicine | 10–10,000 | 2500, 3500 | 91.8–102.2 | HPLC-DAD | [34] |
| Material | Target Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (µg L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| Cu@graphitic carbon cages | fluoroquinolones | food, environmental | 0.1–500 1–500 b | 0.018–0.042 0.61–1.76 b | 81.3–104.3 | HPLC-UVD | [62] |
| carboxylated ZIF-8 | methamphetamine | biological | 50–2500 | 10 | 99.83 | HPLC-UVD | [63] |
| zinc-based MOF | pesticides | environmental | 0.1–100 | 0.03–0.21 | 91.9–99.5 | GC-FID | [64] |
| HLDH | bisphenol A | environmental | 0.5–200 | 0.12 | 92–97 | HPLC-UVD | [65] |
| NH2-MIL-101 | bisphenols | environmental | 0.05–200 | 0.016–0.131 | 90.8–117.8 | HPLC-FLD | [66] |
| UiO-66 | insecticides | environmental | 10–500 | 0.02–0.4 | 73.7–119.0 | HPLC-MS/MS | [67] |
| Fe3O4@Fe-BTC | blood lipid regulators | environmental | 585–15,400 | 170–467 | 86.7–99 | HPLC-UV/Vis | [68] |
| MOF-5 | thiols | environmental | 0.118–276 | 0.0016–0.0031 | 86.6–98.5 | HPLC-FLD | [69] |
| Material | Target Analyte | Sample Matrix | Linear Range (mg L−1) | Sensitivity a (µg L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| PEI | pesticides | food | - | - | 91–105 | TLC, LC-MS | [71] |
| PANI-NaY | pesticides | food, environmental | 0.05–50 | 1–310 | 64–128 | HPLC-DAD | [72] |
| dPPA | food colorants | food | 100–50,000 b | 0.035–0.055 b | 94.3–102 | FASI-CE-C4D | [73] |
| CDP | quinolones | environmental | 0.025–5 | 2.67–5.50 | 95.47–103.3 | HPLC-UVD | [74] |
| P-N, P-N-F, Si-N, Si-N-F | pyrocatechin, quercetin | food | 1–400 | 50, 80 | 78.06–83.63 | HPLC-UV/Vis | [6] |
| Material | Target Analyte | Sample Matrix | Linear Range (µg L−1) | Sensitivity a (µg L−1) | Recoveries (%) | Detection Method | Ref. |
|---|---|---|---|---|---|---|---|
| PEG@MoS2 | sulfonamides | food | 300–30,000 | 30–200 | 61.80–110.91 | CZE-DAD | [75] |
| MoS2 | sulfonamides | environmental | 500–50,000 | 50–120 | 73.20–111.51 | CZE-DAD | [76] |
| MoS2/CD | flame retardants | environmental | 1–100 | 0.01–0.06 | 80–91 | HPLC | [77] |
| CeO2, ZrO2 | ribose conjugates | biological | - | 0.16–1.59 b | 78.5–97.5 | HPLC-MS/MS | [78] |
| CeO2 | ribose conjugates | biological | - | 4.11–18.09 b | 34.0–55.9 | UHPLC-MS/MS | [79] |
| [C16MIM]Br-AL | insecticides | environmental | 1–500 | 0.14–0.21 | 70.6–97.8 | HPLC-DAD, HPLC-UVD | [80] |
| [C12MIM]Br-ATP | pyrethroids | environmental | 2–500 | 0.3–0.6 | 90.28–107.56 | HPLC-DAD | [81] |
| TFA-TAPB | NAC | environmental | 100–50,000 | 30–90 | 84.0–112.3 | HPLC-DAD | [82] |
| N-Mag-COF | disperse dyes | textile | 0.5–200 c | 0.021–0.058 c | 72.2–107 | UFLC-MS/MS | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścigalski, P.; Kosobucki, P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules 2020, 25, 4869. https://doi.org/10.3390/molecules25214869
Ścigalski P, Kosobucki P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules. 2020; 25(21):4869. https://doi.org/10.3390/molecules25214869
Chicago/Turabian StyleŚcigalski, Piotr, and Przemysław Kosobucki. 2020. "Recent Materials Developed for Dispersive Solid Phase Extraction" Molecules 25, no. 21: 4869. https://doi.org/10.3390/molecules25214869
APA StyleŚcigalski, P., & Kosobucki, P. (2020). Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules, 25(21), 4869. https://doi.org/10.3390/molecules25214869