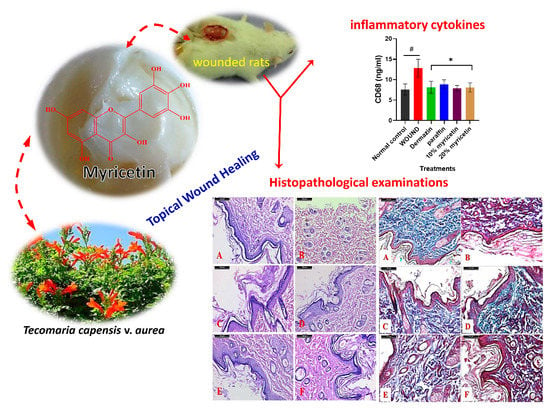
Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Wound Closure Estimation
2.2. Quantitative Analysis of TNF-α and IL1-β
2.3. Effect of Myricetin on CD68 Level in Experimental Rats
2.4. Histological Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials Collection and Preparation
3.3. Extraction, Isolation, and Identification of Myricetin
Physical Properties and Spectroscopic Data of Myricetin
3.4. Experimental Animals
3.5. Ointment Preparation
3.6. Methods Incisional Open Wound Study
3.7. Samples Collection
3.8. Measurement of the TNF-α and IL-1β Level in the Rat Serum
3.9. Estimation of CD68 in the Rat Serum
3.10. Measurement of Wound Diameter and Closure
3.11. Histopathological Examination
3.12. Morphometric Study
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cells Dev. 2019, 28, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Mathias, E.; Srinivas, M.M. Pediatric thermal burns and treatment: A review of progress and future prospects. Medicines 2017, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. 2017, 97, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-L.; Zhao, F.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Song, X.-Y.; Sun, C.-Y.; Yang, J. Promotion of wound healing and prevention of frostbite injury in rat skin by exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef]
- Yeng, N.K.; Shaari, R.; Nordin, M.L.; Sabri, J. Investigation of wound healing effect of Acalypha indica extract in sprague dawley rats. Biomed. Pharmacol. J. 2019, 12, 1857–1865. [Google Scholar] [CrossRef]
- Al-Hussaini, R.; Mahasneh, A.M. Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules 2009, 14, 3425–3435. [Google Scholar] [CrossRef]
- Moussa, A.; Emam, A.; Diab, Y.; Mahmoud, M.; Mahmoud, A. Evaluation of antioxidant potential of 124 Egyptian plants with emphasis on the action of Punica granatum leaf extract on rats. Int. Food Res. J. 2011, 18, 535–542. [Google Scholar]
- Saini, N.K.; Singha, M. Anti–inflammatory, analgesic and antipyretic activity of methanolic Tecomaria capensis leaves extract. Asian Pac. J. Trop. Biomed. 2012, 2, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Magda, T.; Mohamed, M.A.; Manal, M. Cytotoxic activity assesment of secondary metabolites from Tecomaria capensis v. aurea. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1173–1182. [Google Scholar]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Tong, Y.; Lu, S.; Yang, R.; Liao, X.; Xu, Y.-F.; Li, X. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med. 2010, 76, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Rather, I.A. Myricetin abrogates cisplatin-induced oxidative stress, inflammatory response, and goblet cell disintegration in colon of wistar rats. Plants 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.R.; Moon, J.Y.; Ediriweera, M.K.; Song, Y.W.; Cho, M.; Kasiviswanathan, D.; Cho, S.K. Dietary flavonoid myricetin inhibits invasion and migration of radioresistant lung cancer cells (A549-IR) by suppressing MMP-2 and MMP-9 expressions through inhibition of the FAK-ERK signaling pathway. Food Sci. Nutr. 2020, 8, 2059–2067. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Q.; Wu, S.; Yi, D.; Yu, Y.; Liu, S.; Li, S.; Li, Z. Myricetin induces apoptosis via endoplasmic reticulum stress and DNA double-strand breaks in human ovarian cancer cells. Mol. Med. Rep. 2016, 13, 2094–2100. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, H.M.; Ammar, N.M.; Abdelhameed, M.F.; Gendy, A.E.-N.G.E.; Ragab, T.I.; Abd-Elgawad, A.M.; Farag, M.A.; Alwahibi, M.S.; Elshamy, A.I. Protective mechanism of Acacia saligna butanol extract and its nano-formulations against ulcerative colitis in rats as revealed via biochemical and metabolomic assays. Biology 2020, 9, 195. [Google Scholar] [CrossRef]
- Gawish, S.M.; El Din, A.A.G.; Ahmed, H.H.; Farrag, A.R.H.; Abou-El Kheir, A. Effect of newly synthesized polypropylene/silver nonwoven fabric dressing on incisional wound healing in rats. Maced. J. Med. Sci. 2014, 7, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.S.; de Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.M.; Jackson, C.J.; de Azeved, G.L.M. Lupeol, a dietary triterpene, enhances wound healing in streptozotocin-induced hyperglycemic rats with modulatory effects on inflammation, oxidative stress, and angiogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-Y.; Lim, Y. Short term supplementation of dietary antioxidants selectively regulates the inflammatory responses during early cutaneous wound healing in diabetic mice. Nutr. Metab. 2011, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, Z.-X.; Hao, Y.-H.; Gao, Y.-B.; Yao, B.-W.; Zhang, J.; Wang, B.; Hu, Z.-Q.; Peng, R.-Y. A novel microcurrent dressing for wound healing in a rat skin defect model. Mil. Med. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Arulselvan, P.; Ng, S.-F.; Taib, C.N.M.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Grellner, W.; Georg, T.; Wilske, J. Quantitative analysis of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in human skin wounds. Forensic Sci. Int. 2000, 113, 251–264. [Google Scholar] [CrossRef]
- Hübner, G.; Brauchle, M.; Smola, H.; Madlener, M.; Fässler, R.; Werner, S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996, 8, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, I.M.; Tomic-Canic, M.; Komine, M.; Blumenberg, M. Keratins and the keratinocyte activation cycle. J. Investig. Dermatol. 2001, 116, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Gragnani, A.; Müller, B.R.; da Silva, I.D.C.G.; de Noronha, S.M.R.; Ferreira, L.M. Keratinocyte growth factor, tumor necrosis factor-alpha and interleukin-1 beta gene expression in cultured fibroblasts and keratinocytes from burned patients. Acta Cir. Bras. 2013, 28, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Brochhausen, C.; Schmitt, V.H.; Mamilos, A.; Schmitt, C.; Planck, C.N.; Rajab, T.K.; Hierlemann, H.; Kirkpatrick, C.J. Expression of CD68 positive macrophages in the use of different barrier materials to prevent peritoneal adhesions—An animal study. J. Mater. Sci. Mater. Med. 2017, 28, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chou, H.; Li, L.; Li, H.; Cui, Z. Wound healing activity of neferine in experimental diabetic rats through the inhibition of inflammatory cytokines and nrf-2 pathway. Artif. Cells Nanomed. Biotechnol. 2020, 48, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.N.; Masyitha, D.; Harris, A.; Balqis, U.; Iskandar, C.D.; Hambal, M. Anti-inflammatory activity of Jatropha curcas Linn. latex in cream formulation on CD68 expression in mice skin wound. Vet. World 2018, 11, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, G.D.; Whitehead, R.A.; Knighton, D.R. Initiation and pattern of angiogenesis in wound healing in the rat. Am. J. Anat. 1991, 192, 257–262. [Google Scholar] [CrossRef]
- Guo, P.; Feng, Y.-Y. Anti-inflammatory effects of kaempferol, myricetin, fisetin and ibuprofen in neonatal rats. Trop. J. Pharm. Res. 2017, 16, 1819–1826. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.A.; Murillo, R.; Bruhn, T.; Bringmann, G.; Goettert, M.; Heinzmann, B.; Brecht, V.; Laufer, S.A.; Merfort, I. Catechin derivatives from Parapiptadenia rigida with in vitro wound-healing properties. J. Nat. Prod. 2010, 73, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Öz, B.E.; İşcan, G.S.; Akkol, E.K.; Süntar, İ.; Acıkara, Ö.B. Isoflavonoids as wound healing agents from Ononidis Radix. J. Ethnopharmacol. 2018, 211, 384–393. [Google Scholar]
- Muralidhar, A.; Babu, K.S.; Sankar, T.R.; Reddanna, P.; Latha, J. Wound healing activity of flavonoid fraction isolated from the stem bark of Butea monosperma (Lam) in albino wistar rats. Eur. J. Exp. Biol. 2013, 3, 1–6. [Google Scholar]
- Mensah, A.; Sampson, J.; Houghton, P.; Hylands, P.; Westbrook, J.; Dunn, M.; Hughes, M.; Cherry, G. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J. Ethnopharmacol. 2001, 77, 219–226. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. BioMed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Choi, E.M. Myricetin inhibits IL-1β-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int. Immunopharmacol. 2010, 10, 812–814. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Farimani, M.M.; Hamburger, M.; Jabbarzadeh, E. Wound healing potential of chlorogenic acid and myricetin-3-O-β-Rhamnoside isolated from Parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambiga, S.; Narayanan, R.; Gowri, D.; Sukumar, D.; Madhavan, S. Evaluation of wound healing activity of flavonoids from Ipomoea carnea Jacq. Anc. Sci. Life 2007, 26, 45–51. [Google Scholar] [PubMed]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Ram, M.; Kumawat, S.; Tandan, S.; Kumar, D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF-β1. Indian J. Exp. Biol. 2016, 54, 187–195. [Google Scholar] [PubMed]
- Taskan, M.M.; Yuce, H.B.; Karatas, O.; Gevrek, F. Topical quercetin gel application improved wound healing in Wistar rats. Ann. Med. Res. 2019, 26, 2397–2404. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. A hydroxyl group of flavonoids affects oral anti-inflammatory activity and inhibition of systemic tumor necrosis factor-α production. Biosci. Biotechnol. Biochem. 2004, 68, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butenko, I.; Gladtchenko, S.; Galushko, S. Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesisin vitro by flavonoid baicalein from Scutellaria baicalensis georgy roots. Agents Actions 1993, 39, C49–C51. [Google Scholar] [CrossRef]
- Kyo, R.; Nakahata, N.; Kodama, Y.; Nakai, Y.; Kubo, M.; Ohizumi, Y. Antagonism of saikosaponin-induced prostaglandin E2 release by baicalein in C6 rat glioma cells. Biol. Pharm. Bull. 1999, 22, 1385–1387. [Google Scholar] [CrossRef] [Green Version]
- Krakauer, T.; Li, B.Q.; Young, H.A. The flavonoid baicalin inhibits superantigen-induced inflammatory cytokines and chemokines. FEBS Lett. 2001, 500, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Gomathi, K.; Gopinath, D.; Ahmed, M.R.; Jayakumar, R. Quercetin incorporated collagen matrices for dermal wound healing processes in rat. Biomaterials 2003, 24, 2767–2772. [Google Scholar] [CrossRef]
- Omara, E.A.; El-Toumy, S.A.; Shabana, M.E.; Farrag, A.-R.H.; Nada, S.A.; Shafee, N. The antifibrotic effect of Zilla spinosa extracts targeting apoptosis in CCl4-induced liver damage in rats. J. Arab Soc. Med. Res. 2018, 13, 129–143. [Google Scholar] [CrossRef]
- Jang, J.K.; Lee, O.S.; Kang, T.J.; Lim, S.C. Wound healing effect of cuttlebone extract in burn injury of rat. Food Sci. Biotechnol. 2013, 22, 99–105. [Google Scholar] [CrossRef]
- Amsen, D.; de Visser, K.E.; Town, T. Approaches to Determine Expression of Inflammatory Cytokines. In Inflammation and Cancer: Methods and Protocols: Experimental Models and Practical Approaches; Kozlov, S.V., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 1, pp. 107–142. [Google Scholar]
- Kumar, M.S.; Sripriya, R.; Raghavan, H.V.; Sehgal, P.K. Wound healing potential of Cassia fistula on infected albino rat model. J. Surg. Res. 2006, 131, 283–289. [Google Scholar] [CrossRef]
- Drury, R.; Wallington, E. Carleton’s Histological Technique, 5th ed.; Oxford University Press: Oxford, UK, 1980. [Google Scholar]




| Group | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| Wound | 101.5 ± 1.1 | 82.5 ± 3.2 (18.7%) a | 33.1 ± 2.7 (67.3%) |
| Dermazin | 115.3 ± 5.3 | 36.7 ± 1.6 * (63.8%) | 2.7 ± 1.8 * (97.3%) |
| Paraffin | 103.5 ± 2.7 | 54.5 ± 3.4 * (46.3%) | 3.7 ± 2.2 * (96.3%) |
| 10% Myricetin | 113.8 ± 2.2 | 47.2 ± 6 * (53.4%) | 11.0 ± 2.3 * (89.1%) |
| 20% Myricetin | 111.3 ± 2.8 | 34.1 ± 2.7 * (66.4%) | 1.2 ± 0.9 * (98.8%) |
| Groups | TNF-α | IL-1β |
|---|---|---|
| Normal control | 76.2 ± 8.2 | 86.1 ± 7.7 a |
| Wound | 668.6 ± 49.2 * | 616.3 ± 47.6 * |
| Dermazin | 116.6 ± 13.9 ** | 131.4 ± 13.2 ** |
| Paraffin | 126 ± 14.2 ** | 148.9 ± 7.8 ** |
| 10% Myricetin | 110.7 ± 12.9 ** | 136.3 ± 21.7 ** |
| 20% Myricetin | 77.3 ± 3.8 ** | 96.3 ± 5.7 ** |
| Group | Area of Collagen (µm²) |
|---|---|
| Normal | 48 ± 4.5 a |
| + Control | 28.7 ± 2.4 * |
| 10% Myricetin | 45.1 ± 5.9 ** |
| 20% Myricetin | 54.8 ± 1.8 ** |
| Drug | 43 ± 5.5 ** |
| Paraffin | 25.5 ± 4.9 * |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; El-Kashak, W.A.; Al-Rejaie, S.S.; Abd-ElGawad, A.M.; Farrag, A.-R.H. Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea. Molecules 2020, 25, 4870. https://doi.org/10.3390/molecules25214870
Elshamy AI, Ammar NM, Hassan HA, El-Kashak WA, Al-Rejaie SS, Abd-ElGawad AM, Farrag A-RH. Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea. Molecules. 2020; 25(21):4870. https://doi.org/10.3390/molecules25214870
Chicago/Turabian StyleElshamy, Abdelsamed I., Naglaa M. Ammar, Heba A. Hassan, Walaa A. El-Kashak, Salim S. Al-Rejaie, Ahmed M. Abd-ElGawad, and Abdel-Razik H. Farrag. 2020. "Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea" Molecules 25, no. 21: 4870. https://doi.org/10.3390/molecules25214870
APA StyleElshamy, A. I., Ammar, N. M., Hassan, H. A., El-Kashak, W. A., Al-Rejaie, S. S., Abd-ElGawad, A. M., & Farrag, A. -R. H. (2020). Topical Wound Healing Activity of Myricetin Isolated from Tecomaria capensis v. aurea. Molecules, 25(21), 4870. https://doi.org/10.3390/molecules25214870