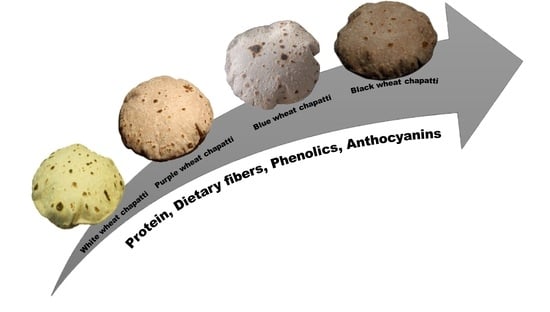
Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Grain Characteristics
2.2. Dough Extensibility and Chapatti Separation Distance
2.3. Color Measurement of Flour and Chapattis
2.4. Sensory Evaluation of Chapattis
2.5. Proximate Analysis of Whole Wheat Flours and Chapattis
2.6. Phenolic Compounds and Anthocyanin Content of Flour and Chapatti
2.7. Antioxidant Activity of Flour and Chapatti
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Wheat Flour
3.3. Physico-Chemical Properties of Wheat
3.4. Chapatti Preparation
3.5. Proximate Analysis of Flours and Chapatti
3.6. Physical Characteristics of Chapattis
3.6.1. Texture and Dough Extensibility
3.6.2. Color Analysis
3.6.3. Sensory Evaluation of Chapatti
3.7. Determination of Phytochemical and Antioxidant Activity
3.7.1. Soluble Phenolic Content
3.7.2. Insoluble Phenolic Content
3.7.3. Total Anthocyanin Content
3.7.4. DPPH Radical Scavenging Activity
3.7.5. ABTS Radical Cation Decolorization Assay
3.7.6. PCL Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Chunduri, V.; Kumar, A.; Kumar, R.; Khare, P.; Kondepudi, K.K.; Garg, M. Anthocyanin bio-fortified colored wheat: Nutritional and functional characterization. PLoS ONE 2018, 13, e0194367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansakova, K.; Babickova, J.; Havrlentova, M.; Hodosy, J.; Kraic, J. The effects of anthocyanin rich wheat diet on the oxidative status and behavior of rats. Croat. Med. J. 2016, 57, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, J.; Yue, Y.; Li, K.; Ren, G. Dietary black-grained wheat intake improves glycemic control and inflammatory profile in patients with type 2 diabetes: A randomized controlled trial. Ther. Clin. Risk Manag. 2018, 14, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-biofortified Colored Wheat Prevents High Fat Diet-induced Alterations in Mice: Nutrigenomics Studies. Mol. Nutr. Food Res. 2020, 64, 1900999. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.S.; Manohar, R.S.; Salimath, P.V.; Rao, U.J.P. Effect of added arabinoxylans isolated from good and poor chapati making wheat varieties on rheological properties of dough and chapati making quality. Food Nutr. Sci. 2013, 4, 884. [Google Scholar] [CrossRef] [Green Version]
- Panghal, A.; Chhikara, N.; Khatkar, B.S. Characterisation of Indian wheat varieties for chapatti (flat bread) quality. J. Saudi Soc. Agric Sci. 2019, 18, 107–111. [Google Scholar] [CrossRef]
- Gujral, H.S.; Singh, G.S.; Rosell, C.M. Extending shelf life of chapatti by partial baking and frozen storage. J. Food Eng. 2008, 89, 466–471. [Google Scholar] [CrossRef]
- Martinek, P.; Skorpik, M.; Chrpova, J.; Schweiger, J.F.P. Development of the new winter wheat variety Skorpion with blue grain. Czech J. Genet. Plant Breed. 2013, 49, 90–94. [Google Scholar] [CrossRef] [Green Version]
- CIMMYT. Wheat Research and Development in Pakistan; CIMMYT: Mexico City, Mexico, 1989. [Google Scholar]
- Kassegn, H. Determination of proximate composition and bioactive compounds of the Abyssinian purple wheat. Cogent Food Agric. 2018, 4, 1421415. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Xu, P.; Guo, Y. Analysis of nutrient composition of purple wheat. Cereal Res. Commun. 2013, 41, 293–303. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; De Leonardis, A.M.; Giovanniello, V.; Del Nobile, M.A.; Padalino, L.; De Vita, P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in human health. Int. J. Chem. Eng. Appl. 2014, 5, 393. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Roy, M.K.; Takenaka, M.; Isobe, S.; Tsushida, T. Antioxidant potential, anti-proliferative activities, and phenolic content in water-soluble fractions of some commonly consumed vegetables: Effects of thermal treatment. Food Chem. 2007, 103, 106–114. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. 2015. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Beta, T. Flour and bread from black-, purple-, and blue-colored wheats. In Flour and Breads and Their Fortification in Health and Disease Prevention, 2nd ed.; Victor Preedy, V., Watson, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 59–67. [Google Scholar]
- Alavi, S.; Giannetta, F.; Nanjundaswamy, A.; Madl, R.; Vadlani, P. Delivery of antioxidants through fruits and vegetables in extruded foods. Cereal Foods World 2014, 59, 179–185. [Google Scholar] [CrossRef]
- Garg, M.; Tsujimoto, H.; Gupta, R.K.; Kumar, A.; Kaur, N.; Kumar, R.; Chunduri, V.; Sharma, N.K.; Chawla, M.; Sharma, S.; et al. Chromosome specific substitution lines of Aegilops geniculata alter parameters of bread making quality of wheat. PLoS ONE 2016, 11, e0162350. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Tanaka, H.; Ishikawa, N.; Takata, K.; Yanaka, M.; Tsujimoto, H. Agropyron elongatum HMW-glutenins have a potential to improve wheat end-product quality through targeted chromosome introgression. J. Cereal Sci. 2009, 50, 358–363. [Google Scholar] [CrossRef]
- Kadam, M.L.; Salve, R.V.; Mehrajfatema, Z.M.; More, S.G. Development and evaluation of composite flour for Missi roti/chapatti. J. Food Process. Technol. 2012, 3, 134–140. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Method of Analysis, 13th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of total, soluble, and insoluble, dietary fiber in foods-enzymatic-gravimetric method, MES-TRIS buffer: Collaborative study. J. Assoc. Off. Anal. Chem. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Kumar, A.; Garg, M.; Kaur, N.; Chunduri, V.; Sharma, S.; Misser, S.; Kumar, A.; Tsujimoto, H.; Dou, Q.W.; Gupta, R.K. Rapid development and characterization of chromosome specific translocation line of Thinopyrum elongatum with improved dough strength. Front. Plant Sci. 2017, 8, 1593. [Google Scholar] [CrossRef] [Green Version]
- Gurushree, M.N.; Nandini, C.R.; Pratheeksha, K.; Prabhasankar, P.; Hosamane, G.G. Design, development and performance evaluation of chapati press cum vermicelli extruder. J. Food Sci. Technol. 2011, 48, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Kannojiya, G.; Kumar, A.; Gaur, A.K. Assay of Anti-Oxidative Activity of Seed Extracts Derived from Rice (Oryza sativa L.) Varieties. Int. J. Curr. Microbiol. Appl. Sci. 2017, 8, 2659–2666. [Google Scholar] [CrossRef] [Green Version]
- Young, J.C.; Abdel-Aal, E.M. Anthocyanidins. In Analysis of Bioactive Components in Small Grain Cereals; Shewry, P.R., Ward, J.L., Eds.; AACC International Press: St. Paul, MN, USA, 2010; pp. 141–165. [Google Scholar]



| Sample | Grain Hardness Index | Kernel Diameter (mm) | TKW (g) | Test Weight (Kg/hl) | SDSS (cm) | Chapatti Separation Distance (mm) | Dough Extensibility | |
|---|---|---|---|---|---|---|---|---|
| Force (gm) | Distance (cm) | |||||||
| WW | 76.22 ± 2.15 a | 3.04 ±0.06 b | 45.18 ± 2.00 a | 1191.5 ± 16.71 a | 8.96 ± 0.15 a | 7.81 ± 1.59 a | 27.11 ± 4.27 a | 1.84 ± 0.23 a |
| PW | 84.91 ± 0.41 b | 3.18 ± 0.02 c | 49.29 ± 0.86 b | 1192.39 ± 0.68 a | 7.73 ± 0.21 b | 8.72 ± 1.87 a | 36.67 ± 5.65 b | 1.91 ± 0.3 a |
| BW | 75.07 ± 0.48 a | 2.77 ± 0.01 a | 43.55 ± 0.60 a | 1174.13 ± 1.10 a | 7.43 ± 0.12 b | 11.36 ± 1.53 b | 20.61 ± 4.51 c | 1.18 ± 0.08 b |
| Bl-W | 76.12 ± 0.71 a | 2.74 ± 0.01 a | 42.7 ± 0.50 a | 1171.76 ± 0.77 a | 6.63 ± 0.32 c | 11.21 ± 1.73 b | 31.33 ± 2.5 a | 1.49 ± 0.14 c |
| Parameters | White Flour | Purple Flour | Blue Flour | Black Flour |
|---|---|---|---|---|
| After Cooking | ||||
| Stickiness | 8.70 ± 0.30 a | 8.20 ± 0.30 a | 9.50 ± 0.50 b | 8.17 ± 0.29 a |
| Water (mL) | 165.0 | 180.0 | 185.0 | 185.0 |
| Roll out Easy (1–10) | 10.0 ± 00 c | 7.30 ± 0.60 a | 9.00 ± 0.00 b | 9.50 ± 0.50 b,c |
| Puffing Height (10) | 6.80 ± 0.70 a | 8.30 ± 0.40 b | 8.67 ± 0.41 b | 8.92 ± 0.38 b |
| Aroma (10) | 8.40 ± 0.50 b | 8.30 ± 0.50 a,b | 8.11 ± 0.55 a,b | 7.61 ± 0.86 a |
| Mouth feel (10) | 8.80 ± 1.10 a | 8.50 ± 1.10 a | 8.83 ± 0.61 a | 8.06 ± 1.24 a |
| Taste (10) | 8.60 ± 1.50 a | 8.50 ± 0.90 a | 8.56 ± 1.33 a | 7.83 ± 1.54 a |
| Appearance (10) | 8.00 ± 1.20 a | 8.10 ± 1.00 a | 8.11 ± 0.74 a | 7.94 ± 0.63 a |
| Tearing strength (10) | 8.00 ± 1.60 a | 8.10 ± 0.70 a | 8.33 ± 0.66 a | 7.78 ± 0.71 a |
| After 1 day | ||||
| Roll out (10) | 7.90 ± 0.30 b | 7.10 ± 0.30 a | 8.25 ± 0.29 b | 8.13 ± 0.25 b |
| Puffing Height (10) | 5.30 ± 0.50 a | 8.10± 0.30 c | 8.13 ± 0.25 c | 6.88 ± 0.63 b |
| Taste (10) | 7.80 ± 0.30 a | 7.80 ± 0.30 a | 7.75 ± 0.29 a | 7.75 ± 0.50 a |
| Tearing strength (10) | 7.10 ± 0.30 a,b | 7.60 ± 0.50 b,c | 7.88 ± 0.25 c | 6.50 ± 0.41 a |
| Chapatti Score | 7.90 ± 1.20 | 8.00 ± 0.40 | 8.40 ± 0.50 | 7.90 ± 0.80 |
| S. No | Carbohydrate | Moisture | Fat | Protein | TDF | Ash |
|---|---|---|---|---|---|---|
| WF | 67.91 ± 0.39 a | 10.07 ± 0.31 d | 1.10 ± 0.21 a,b | 9.93 ± 0.11 c | 11.02 ± 0.45 b | 1.67 ± 0.29 a,b |
| PF | 66.03 ± 0.64 b | 9.96 ± 0.22 d | 1.15 ± 0.14 a,b | 11.01 ± 0.02 b | 11.99 ± 0.37 a | 1.88 ± 0.2 a |
| BF | 65.80 ± 0.29 c | 10.01 ± 0.19 d | 1.21 ± 0.19 a | 11.13 ± 0.1 b | 11.85 ± 0.35 a | 1.83 ± 0.29 a |
| Bl-F | 64.43 ± 0.61 c | 9.96 ± 0.24 d | 1.24 ± 0.26 a | 12.14 ± 0.27 a | 12.18 ± 0.4 a | 1.83 ± 0.29 a |
| WC | 47.48 ± 1.04 d | 33.36 ± 1.05 c | 0.85 ± 0.03 a,b | 8.95 ± 0.07 d | 9.36 ± 0.13 d | 1.25 ± 0.02 b |
| PC | 44.98 ± 0.34 e | 34.12 ± 0.31 c | 0.80 ± 0.02 b | 9.94 ± 0.07 c | 10.16 ± 0.11 c | 1.25 ± 0.06 b |
| BC | 40.93 ± 0.52 f | 37.91 ± 0.28 b | 0.84 ± 0.02 a,b | 9.99 ± 0.31 c | 10.33 ± 0.07 c | 1.2 ± 0.00 b |
| Bl-C | 38.24 ± 0.64 g | 39.18 ± 0.6 a | 0.85 ± 0.01 a,b | 10.95 ± 0.39 b | 10.78 ± 0.08 c | 1.38 ± 0.14 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Kaur, S.; Goyal, A.; Garg, M. Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules 2020, 25, 5071. https://doi.org/10.3390/molecules25215071
Kumari A, Sharma S, Sharma N, Chunduri V, Kapoor P, Kaur S, Goyal A, Garg M. Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules. 2020; 25(21):5071. https://doi.org/10.3390/molecules25215071
Chicago/Turabian StyleKumari, Anita, Saloni Sharma, Natasha Sharma, Venkatesh Chunduri, Payal Kapoor, Satveer Kaur, Ajay Goyal, and Monika Garg. 2020. "Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread)" Molecules 25, no. 21: 5071. https://doi.org/10.3390/molecules25215071
APA StyleKumari, A., Sharma, S., Sharma, N., Chunduri, V., Kapoor, P., Kaur, S., Goyal, A., & Garg, M. (2020). Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules, 25(21), 5071. https://doi.org/10.3390/molecules25215071